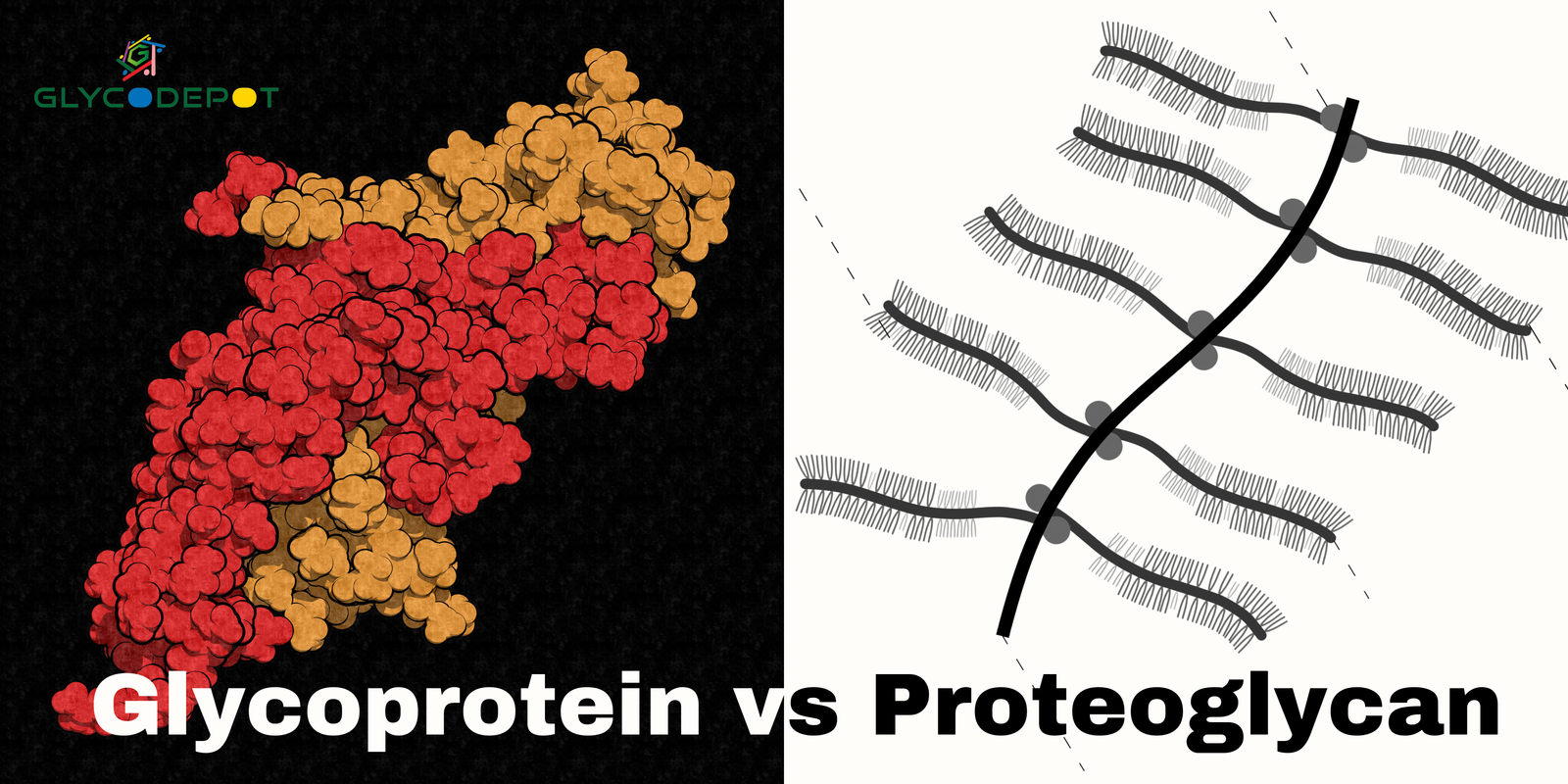
Proteoglycan vs Glycoprotein are integral to the intricate world of cell biology. Both contribute key roles to maintaining critical biological functions, are cellular building blocks, and are fundamental structures in cell communication. Macromolecules are complex carbohydrate-protein complexes that are essential in signaling and cell communication. They are an integral part of the extracellular matrix (ECM).
Despite being relatives, proteoglycans’ distinct physiological roles and structural characteristics distinguish them from glycoproteins. They also emphasize their roles in most physiologic and pathologic processes, such as tissue growth, immunological defense, and disease pathology. By understanding their biological activities and structural complexity, we can better comprehend their role in ECM constituents, cell signaling facilitation, and organismal well-being. Knowing what differentiates Proteoglycan vs glycoprotein, we can better comprehend their distinct roles, biological activity, and significance in human biology.
What Are Proteoglycans?

They convey varied biological activities, particularly structural support and tissue signaling pathways. Proteoglycans are vital to holding the Proteoglycan structure of tissue, hydration, and biochemistry. They have the fundamental function of holding tissue architecture and forming reservoirs of growth factors that regulate cell behavior.
1. Proteoglycan Structure
A prototypic proteoglycan is a highly heterogeneous molecule containing the following components:
- Core protein: The core protein framework attached to the chains of GAG, dictating the molecule size and shape. The core protein itself may also differ in size and complexity and thus impart functional heterogeneity to proteoglycans.
- Linkage region: A tetrasaccharide connector between the GAG chain and the core protein. The linkage binds and orients the GAG chains in a manner that reinforces their biological functions of proteoglycans.
Due to their negatively charged GAG chains and complex Proteoglycan structure, proteoglycans have unusual physical and chemical characteristics. These characteristics allow proteoglycans to create a gel matrix of hydration that confers compressive stiffness and tissue resilience. Proteoglycans’ ability to trap water molecules due to their negatively charged GAG chains also helps tissue’s hydration and mechanical strength.
2. Proteoglycan Examples
Proteoglycans are as varied in function and structure as they regard where they occur and the functions they fulfill within the body. Amongst the most familiar types of proteoglycans are:
- Aggrecan: Aggrecan is among the most extensive proteoglycans of cartilage and is crucial for providing compressive strength to the tissue. Aggrecan aggregates that bind with hyaluronic acid form large aggregates that provide supportive resistance to mechanical stress when the joint is transported.
- Perlecan: It is a crucial proteoglycan present in basement membranes. It confers tissue strength and is involved in cell signaling. Perlecan is linked to many growth factors and enzymes and controls cell behavior and tissue homeostasis.
- Decorin: It is a tissue-localized protein that is bound to collagen fibrils, controlling collagen assembly and tissue structural organization. Decorin regulates growth factor function and, therefore, cell proliferation and differentiation.
- Versican: Versican is present in most tissues, including blood vessels and skin, and is involved in wound and tissue repair. It maintains tissue remodeling to support cell adhesion, migration, and proliferation in harmony.
3. Biological Functions of Proteoglycans
Proteoglycans are involved in numerous biological activities and have such activities as:
Structural support: Proteoglycans confer tissue mechanical stability by assembling as complexes with other molecules of the ECM, such as elastin and collagen. This confers tissue stability and structural support to the system in general, particularly in the connective tissues such as cartilage and skin.
- Hydration: The highly damaging GAG chains have high water molecule affinity and maintain the ECM in a hydrated condition. Hydration is crucial to impart flexibility and elasticity to tissues, particularly in compressive force-transmitting tissues such as cartilage of joints.
- Molecular sieving: Proteoglycans also control the diffusion of molecules across tissues. Their ability to establish a selective barrier allows the movement of some molecules but not others, thereby influencing tissue permeability and molecular transport.
Binding to growth factors: Proteoglycans serve as reservoirs for growth factors and other signaling molecules. They can regulate activity and availability by binding growth factors, which regulate cellular communication molecules involved in tissue repair, development, and immunity.
What are Glycoproteins?

The cell membrane contains proteins free to wander within or adjacent to it. They can move and interact with the cell environment. In science, the prefix “glyco” denotes “sugar.” Either the reaction is performed during protein translation or post-translation by glycosylation.
Glycoproteins result when the protein moiety is in a greater quantity than the carbohydrate-protein ratio. It is called a proteoglycan if excess carbohydrates characterize the complex compared to proteins. Glycolipids are the result of the combination of carbohydrates and lipids.
1. Glycoprotein Structure
Glycoproteins have the following principal structural components:
- Protein core: The protein component is the central structural element of glycoproteins and constitutes most of their total structure. The core gives the molecule the structural and functional basis to carry out certain glycoprotein functions, depending on its location and function in the body.
- Oligosaccharide chains: Oligosaccharide chains are the shorter, typically branched chains of carbohydrates covalently bonded to proteins. Significant roles are played by oligosaccharides in regulating the characteristics of glycoproteins, including their recognition, binding, and resistance to degradation. Glycoproteins’ dense carbohydrate chains are just as important to biology as the longer chains of proteoglycans.
- Linkage: The carbohydrate chains are joined to the protein backbone by N-linked (to the amino acid asparagine) or O-linked (to serine or threonine) glycosidic connections. The linkages depend upon proper folding, stability, and interaction with other molecules. The glycosidic bond type, either N-linked or O-linked, governs carbohydrate attachment type and can influence glycoprotein function.
2. Glycoprotein Examples
Glycoproteins occur all over the biological system and are involved in many processes. A few glycoproteins are examples.
- Antibodies (Immunoglobulins): They are significant glycoproteins with vital roles in the immune system. They attach to and recognize foreign invading pathogens, e.g., bacteria and viruses. Antibody glycosylation is essential in their stability and as interacters in the immune cells.
- Hormones: Some hormones, such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), are glycoproteins. They regulate critical reproductive functions, such as ovulation and spermatogenesis, by binding to specific target cell receptors. Glycosylation of the hormones regulates their half-life and receptor binding.
- Enzymes: Some enzymes, such as glycosylated transferases and hydrolases, depend on glycosylation for folding, stability, and catalysis. Glycosylation may influence how enzymes regulate reactions and are regulated in cells.
- Cell surface receptors: Cell surface glycoprotein receptors, including growth factor receptors (e.g., epidermal growth factor receptor), convey cell responses to extracellular stimuli, including growth, differentiation, and survival. Oligosaccharide chains of receptors determine their ligand binding specificity and downstream signal transduction pathways.
3. Biological Role of Glycoproteins
Glycoproteins are involved in numerous biological processes and are needed for cells, tissue, and the immune system to operate. Protein stability, targeting, and interaction with other molecules are glycosylation of glycoprotein carbohydrates. Some of the most critical biological activities of glycoproteins include:
- Cell recognition and signaling: Glycoproteins are cell surface receptors and markers that allow cells to sense their environment and respond and sense adjacent cells. For example, surface glycoproteins of capable immune cells will allow them to sense and respond to infection. Glycoproteins must also be crucial in interneuronal communication and intrasynaptic transmission within the nervous system.
- Immune response: Most immune molecules, such as antibodies and major histocompatibility complex (MHC) proteins, are glycoproteins. Glycosylation is necessary to respond to and recognize pathogens appropriately and differentiate self from non-self molecules.
- Enzyme activity: Glycosylation can significantly influence enzyme activity by modifying its stability, activity, and degradability. In some instances, glycosylation must be done before the enzyme achieves its active conformation, but it is also used in enzyme-substrate interaction regulation.
- Lubrication: Mucin glycoproteins lubricate surfaces such as the intestinal and respiratory tracts. Their carbohydrate groups allow mucins to hold water and form a thick barrier that covers tissues and maintains them from mechanical trauma and microorganism invasion.
Key Distinctions Between Glycoprotein and Proteoglycan

It is necessary to define proteoglycan and glycoprotein structural and functional differences so their contribution to cell processes and human physiology is achieved. Although carbohydrates are made of carbohydrates attached to a protein background, the latter defines them based on carbohydrate organization, space organization, and biological processes. All the differences constitute the basis of their functionalities within tissues, cells, and pathologies.
1. Structural Differences
Structural variations in proteoglycan vs glycoprotein influence their biological activity and sites of local occurrence in the organism. Each molecule has developed based on its functions in pathological and physiological systems.
Carbohydrate Composition
- Proteoglycans: Proteoglycans are protein molecules found almost entirely in tissues’ extracellular matrix (ECM). They are structural supporters by binding to other ECM components, such as collagen and elastin. These compounds also help regulate the tissue’s water content, bind growth factors, and influence how cells migrate and repair tissues. Proteoglycans contribute significantly to the mechanical strength and elasticity of highly structured tissues like cartilage and other connective tissues.
- Glycoproteins: While glycolipids contain a higher ratio of carbohydrates, glycoproteins contain a lesser ratio, in most cases, only 1-60% of the molecule. Protein is preponderant in glycoproteins, and hence, glycoproteins can perform functions such as cell signaling and immune recognition.
Core Protein Size
- Proteoglycans: They typically possess gigantic core proteins that serve as a matrix upon which other long GAG chains are attached. Proteoglycans gain the weight and high charge required for mechanical and signaling roles in the extracellular matrix.
- Glycoproteins: Glycoproteins typically contain fewer core proteins than proteoglycans. The structures of the smaller proteins are more stable and valuable since they have to be involved in various processes, including hormone signaling and enzyme regulation.
2. Carbohydrate Chain Structure
- Proteoglycans: Proteoglycan carbohydrate chains are linear extended GAG chains. More precisely, proteoglycans are a repeat of disaccharide units of GAG to create a linear molecule, which gives structural uniformity and water content to tissue.
- Glycoproteins: Glycoproteins comprise branched short oligosaccharides of complex, branched molecules of more significant heterogeneity. Glycoproteins are used in cell-cell recognition, immunocommunication, and protein stabilization.
3. Structure of Carbohydrate Chains
Carbohydrate chains within proteoglycan vs glycoprotein structure give them specific functions.
- Proteoglycans: Keratan sulfate, chondroitin sulfate, and heparan sulfate are some examples of GAG chains, which are carbohydrate chains within proteoglycans. GAGs are heavily negatively charged as they contain uronic acid and sulfate groups. Thus, they can bind water and ions, hydrate, and mechanically stabilize the tissue.
- Glycoproteins: Monosaccharides such as galactose, mannose, and fucose constitute the oligosaccharide chains that comprise glycoproteins. Because of their neutral or negatively charged chains, glycoproteins can be involved in immunological recognition, cell signaling, and receptor-ligand interactions, among other molecular interactions.
4. Biological Functions and Locations
Structural variations in proteoglycan vs glycoprotein structures have their equivalent in their unique biological functions and sites in the body.
- Proteoglycans: These proteins occur exclusively in the extracellular matrix (ECM) and support tissue structure by binding to other ECM molecules, such as collagen and elastin. They also control the amount of water, bind growth factors, and control cell migration and tissue repair. Proteoglycan function is significant in highly structured tissues, such as cartilage and connective tissue.
- Glycoproteins: Proteins usually found on the cell surface, in extracellular fluid, and inside cells, functioning as receptors, enzymes, or messengers. Glycoproteins are vital in cell recognition, immunity, and signal transmission. Glycoproteins also play essential roles in endocrine regulation and immune surveillance.
Functions and Importance in Human Biology
Glycoproteins and proteoglycans have a wide range of functions in human biology but differ depending on their specific structures and locations.
Role in Tissue Development and Tissue Repair
- Proteoglycans: Proteoglycans play a role in tissue development and repair, mainly cartilage and bone development. They are also in charge of the mechanical properties of the tissues, such as compressive strength and elasticity, which tissues require for tissue regeneration and wound healing.
- Glycoproteins: Glycoproteins are responsible for cell migration, adhesion, and angiogenesis on the contrary. These activities are particularly crucial in tissue repair after injury, where glycoproteins control the movement of cells and create new capillaries to rebuild tissue function.
Contribution to Immune Responses
- Proteoglycans: Proteoglycans control immune responses by interacting with cytokines and controlling the migration of immune cells like leukocytes. The interaction controls inflammation and tissue repair after injury or infection.
- Glycoproteins: Glycoproteins have even more immediate roles in the immune system. Antibodies and major histocompatibility complex (MHC) proteins are two glycoproteins essential for immune recognition and response. Their glycosylation patterns enable immune cells to recognize self versus non-self and develop an effective immune response.
Relevance in Disease Mechanisms
Dysregulation of proteoglycan vs glycoprotein expression or function underlies most diseases, including cancer, autoimmune disease, and genetic syndromes.
- Cancer: Proteoglycan and glycoprotein structure changes can facilitate tumor growth, metastasis, and drug resistance. Some proteoglycans have been found to alter the tumor microenvironment, whereas changes in glycoprotein glycosylation can be involved in tumor cells’ immune evasion.
- Autoimmune diseases: Abnormal glycoprotein glycosylation can result in an autoimmune attack, in which the immune system attacks the body’s cells. Glycoprotein structural changes are observed in rheumatoid arthritis and lupus.
- Genetic disorders: Defects in proteoglycan or glycoprotein synthesizing lead to congenital disorders such as Marfan syndrome or Ehlers-Danlos syndrome. Such disorders impair connective tissue integrity and manifest in joints, skin, and cardiovascular systems.
Clinical and Medical Relevance
Impact on Connective Tissue Diseases
- Proteoglycans: As components of tissues, proteoglycans are highly significant to the structural integrity of tissues. Proteoglycan degradation, particularly in osteoarthritis and other disorders, results in cartilage degeneration, pain, and restricted mobility of joints.
- Glycoproteins: Glycoproteins play very critical roles in immune disease and cancer. Disease mechanisms are controlled by glycosylation modifications that modulate immune cells’ reaction to pathogens or tumor cells.
Therapeutic Applications and Targeting of Drugs
Proteoglycan and glycoprotein biology have opened up therapeutic opportunities:
- Drug targeting: Glycoproteins, via their typical binding to specific cell types (e.g., on cancer cells), are targets for anticancer drug targeting antibody-drug conjugates, enabling the targeted treatment of cancer cells using cytotoxic drugs.
- Enzyme replacement therapy: Gaucher’s disease, among other genetic diseases, is treated by infusing patients with recombinant glycoproteins. These glycoproteins replace absent enzymes and facilitate the breakdown of particular compounds.
- Anti-inflammatory drugs: Modulating cytokine-proteoglycan interaction can prevent inflammation in disease states like rheumatoid arthritis and offer novel avenues for anti-inflammatory therapy.
We must be aware of these differences: Proteoglycan vs glycoprotein are structurally different and have different underlying health and disease functions. Awareness of these differences is essential to constructing new therapeutic schemes for tissue degradation to cancer conditions.
Proteoglycan vs Glycoprotein Synthesis: A Comparison Table
| Characteristic | Proteoglycans | Glycoproteins |
|---|---|---|
| Carbohydrate content | High (up to 95% | Lower (typically 1 60% |
| Core protein size | Often large | Generally smaller |
| Carbohydrate structure | Long, unbranched GAG chains | Shorter, often branched oligosaccharides |
| Main location | Extracellular matrix | Cell surfaces, extracellular fluids, intracellular |
| Primary functions | Structural support, hydration, molecular filtering | Cell recognition, signaling, enzymatic activity |
| Examples | Aggrecan, perlecan, decorin | Antibodies, hormones, enzymes |
| Synthesis | Golgi apparatus | Endoplasmic reticulum and Golgi apparatus |
Conclusion
Glycoproteins and proteoglycans are two massive carbohydrate protein interaction complexes that differ in structural elements and biological functions. Their functions in human physiology distinguish them. Proteoglycans stabilize the extracellular matrix structural framework with their long glycosaminoglycan (GAG) chains and offer space for extensive molecular interactions. Conversely, Glycoproteins use their branched, shorter oligosaccharides to perform necessary functions like cellular signaling, immune system recognition, and enzymatic activities.
Ongoing research into these multi-functional molecules only serves to elucidate their functions in disease and health further. The research development will undoubtedly translate into new therapeutic and diagnostic modalities for proteoglycan and Glycoprotein function. Some of the potential areas of future research are:
- Therapeutic development targeting specific proteoglycan or glycoprotein alterations in autoimmune disease and cancer.
- Exploring the potential applications of engineered glycoproteins and proteoglycans in tissue engineering and regenerative medicine.
- Exploring their uses in immunotherapy and personalized medicine, offering more precise ways of addressing complex diseases.
By interpreting the complexity of proteoglycan and glycoprotein biology, researchers add depth to our knowledge of protein and carbohydrate control of cellular interaction, tissue form, and disease pathology. This knowledge has tremendous potential to change how treatments are developed and propel medical science in oncology, immunology, and regenerative biology.
Some of the information above is used from www.glycoforum.gr.jp/glycotext/index.html