At Glycodepot, we pride ourselves on supplying high-quality α-D-Fructofuranose, sourced and processed to fulfil the harsh requirements of research laboratories and industrial applications. Our product has the highest purity and consistency levels, and secure, reliable performance in any application.
Significance in Research and Industry
The unique structure and reactivity, α-D-Fructofuranose make it a central player in glycochemistry and carbohydrate research. Building blocks such as α-D-Fructofuranose are key components for the development of different carbohydrate derivatives; as an example there are some enzymatic methods developed for the preparation of regio-protected D-fructose derivatives such as the simple sugar D-psicose; there are important suggestions for pharmaceutical and nutraceutical sectors with these rare sugars so the value of α-D-Fructofuranose in such advanced research context is significant.
In food production, α-D-Fructofuranose derivatives have been widely studied for the nutritional content of foods. In a case study, an exciting use of a new D-fructofuranose 1,2′:2,3′-dianhydride hydrolase (DFA-IIIase) was used for burdock root to produce functional biomaterials; inulobiose was produced as one such example. Enzymes are capable of improving food products, also the example of D-fructofuranose derivatives can be used to environmentally improve nutrition, functionality, or processing of food products.
Standing Firm on Quality
Glycodepot recognizes the importance of α-D-Fructofuranose in many industries and commits to quality. The product we have chosen will not only be reliable, but also will be thoroughly documented with Certificates of Analysis (CoA) to help you feel confident that it is pure and appropriate for your applications. By selecting Glycodepot’s α-D-Fructofuranose, researchers and industry leaders can take on a greater workflow, with peace of mind knowing they have a consistent and valuable, reliable, and quality product with them.
What is α-D-Fructofuranose?
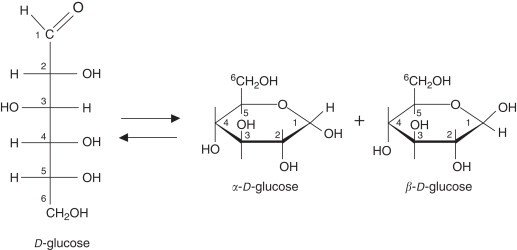
α-D-Fructofuranose, the cyclic form of the monosaccharide D-fructose, is a five-membered ring, known as a furanose. This is a form where the carbonyl functional group on C-2 of the linear fructose molecules reacts with the hydroxyl group on C-5 to form a hemiacetal. The “α” describes the orientation of the hydroxyl group on the anomeric carbon, which is C-2, to be on the opposite side of the ring to the CH₂OH group on C-5.
In aqueous solution, D-fructose is in equilibrium among multiple isomers: α-fructofuranose, β-fructofuranose, and β-fructopyranose. The ratio of these isomers will vary depending on the temperature and solvent. Due to the properties of the solvent, in aqueous-ethanol solutions, the equilibrium shifts to favor furanose stability and has a substantial amount of α-D-fructofuranose included in the solution. This is influenced by the solvent’s effect on the mutarotation kinetics of fructose.
The mutarotation is the conversion of its anomeric forms. Knowing the structural details of α-D-fructofuranose is important to glycochemistry as fructofuranose helps determine the reactivity of all kinds of carbohydrates because of its impact on the formation of glycosidic bonds (gaining structural strength/functionality). Because of the orientation of the hydroxyl groups, the resultant molecule will affect reactivity, and specific reactions of carbohydrates with enzymatic impedances can inherently impact each carbohydrate’s function within biological systems.
For many researchers or industries collaborating with carbohydrates, knowing the properties of α-D-fructofuranose is a priority for the functional basis of its presence in solution. Solvent and solution dynamics will affect crystals, sweetening effects, functional food development, and shaping the properties of pharmaceutical builds.
What Are Monosaccharides and Why Are They Important in Glycochemistry?
Monosaccharides are the most basic unit of carbohydrates, typically represented by the formula (CH2O)n, where n represents the number of sugar units. They form the structural basis for more complex carbohydrates such as disaccharides, oligosaccharides, and polysaccharides. Monosaccharides are also necessary for glycochemistry, the study of the chemical properties of sugars and their derivative. In studying monosaccharides, their structure, reactivity, and functional roles in biological and synthetic applications are important.
They are composed of various carbon chains and stereochemistry because of the numerous potential configurations of hydroxyl groups based on the asymmetric carbon atom arrangements. Their variability provides them the opportunity to act as starting points in polysaccharide synthesis of glycosylated polymers that perform roles in biological processes such as cell signaling, protein folding, immune recognition, and energy metabolism.
In terms of chemical synthesis, the basic scaffolds of monosaccharides allow for the formation of glycosidic bonds in the oligosaccharides and glycoprotein chain scaffolds. The ability to protect and selectively activate specific monosaccharides is sometimes challenging and is especially true in a glychemical context where replication of the selectivity that comes from biological origins is needed.
More recently, the pharmaceutical and biotechnology industries have begun to take note of this field of research and have used this knowledge to produce glycosylated drugs that conjugate sugars to small molecules and/or proteins to increase structural stability and the biological effects of drugs.
Buy Different Types of α-D-Fructofuranose at Glycodepot
Glycodepot offers a wide variety of α-D derivatives for research and industry purposes. Products are available as monomers and more structurally complex derivatives (difructose anhydrides (DFA), α-D-fructofuranosyl-glucose derivatives). These specialized saccharides are made using enzymatic and thermal treatment processes that provide high purity of the product and a specific structure.
For example, difructose anhydrides such as DFA III ( α-D-fructofuranose-β-D-fructofuranose 1,2′:2,3′-dianhydride) are produced via enzymatic action using inulin fructotransferase. The DFAs are low-calorie functional disaccharides, are approximately half as sweet as sucrose, and have about 1/15 the calorie content of sucrose that is not digested in the gastrointestinal area, thus making them an appealing sugar substitute for health-conscious consumers.
DFA III allows the bacteria to increase rapidly in the stomach, promoting a healthier gastrointestinal system. Several publications underscored the structural confirmation and possible synthesis pathways of DFAs have made findings to support similar claims that all DFAs and α-D-fructofuranosyl-glucose derivative products are relevant for developing functional foods for gastrointestinal health.
Moreover, α-D-fructofuranosyl-(2→6)-D-glucose, a disaccharide produced in the fermentation of fruit and vegetable extracts, has been identified and studied for its interesting characteristics. This disaccharide is produced under specific thermal conditions, isolated, and confirmed using suitable techniques, highlighting its relevance in the fields of food science and nutrition. Many studies established that this disaccharide has a low digestibility and is non-carcinogenic, which may provide an opportunity for establishing functional foods in human nutrition to promote gut health. Furthermore, this disaccharide has been shown to feed probiotic bacteria, such as Bifidobacterium species, providing evidence that it can be beneficial as a substrate prebiotic.
At Glycodepot, we understand the importance of quality. Each product is subject to stringent testing and quantification to comply with the regulatory and industrial standards encountered in high-end research and job-related tasks. All of the specialized α-D-fructofuranose derivatives provided through Glycodepot represent potential contributions to knowledge and research in glycochemistry, food technology, and drug development.
Types of α-D-Fructofuranose
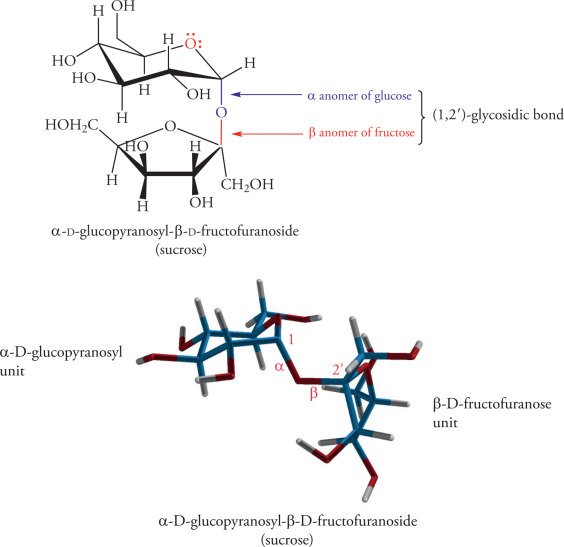
Fig 28.11. Structure of types of alpha-D Fructofuranose.
Source: Adapted from Science Direct.
What is the α-D-Fructofuranose Structure?
The Fructofuranose molecule has a five-membered ring, made up of four carbons and one oxygen, which constitutes its furanose ring. The configuration at the anomeric carbon (carbon 2) distinguishes the α-anomer from the β-anomer; if the hydroxyl group on the anomeric carbon is opposite to the CH₂OH group at carbon 5, it is designated as the α-anomer. This configuration can have an impact on reactivity and subsequent glycosidic linkages in chemically functional molecules.
Chemical information about α-D-Fructofuranose is important so that it can be used effectively in synthetic chemistry or biochemistry applications. The ring form of α-D is stabilized by intramolecular hydrogen bonding, and the flexibility of the ring allows it to be involved in most glycosylation reactions. Thus, it can easily be used in carbohydrate drug discovery, enzyme-substrate assessments, or functional food ingredients.
Structural information has been obtained from crystallography, such as crystallography by Rao et al. (1998) that force significant high-resolution structural data for α-D-fructofuranose, and helped with molecular conformational design for more realistic models of carbohydrates in a reaction, or reagents.
Structural Representation of alpha-D Fructofuranose
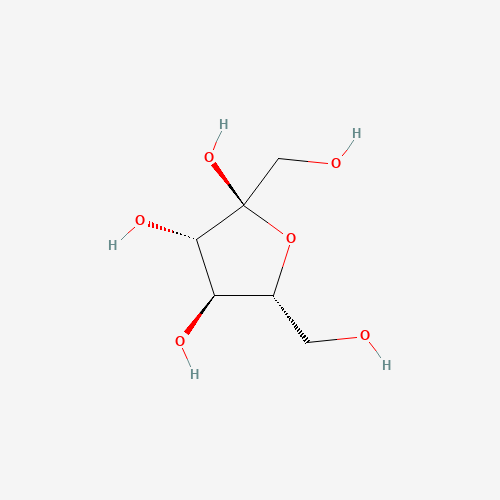
Fig.1. Structural representation of Alpha-D-Fructofuranose.
Source: Adapted from PubChem.
Examples of α-D-Fructofuranose
α-D-Fructofuranose is not only bound to one molecular identity; rather, it appears in several different natural and synthetic carbohydrate structures. One important example is sucrose, a disaccharide formed from the glycosidic bond between α-D-glucose and α-D. This bond and the subsequent relationship that α-D-fructofuranose has with sucrose as a whole enable its sweetening and stability. Another interesting example is inulin-derived difructose anhydride III (DFA III). As with sucrose, DFA III includes two fructose molecules that are joined by a dianhydride bridge with α-D-fructofuranose as one of the core structures.
In addition to sucrose and DFA III, many plant-based oligosaccharides contain α-D-fructofuranose within their structures, such as compounds called 1-kestose and nystose, containing two fructofuranose units that are bonded with a β(2→1) glycosidic bond. These few structures that appeared here provide strong proof that α-D-fructofuranose is a valuable building block in not only dietary sugars but also functional prebiotics. Each of these examples plays a different role in our diets, namely, sucrose as a primary energy source, DFA III as a low-calorie functional sweetener, and the functional oligosaccharides associated with kestose as gut-health-promoting oligosaccharides. Their structural and biochemical properties have been studied from the perspective of human metabolism and for their applicability in food and beverage production.
Structural Representation of Alpha-D Fructofuranose
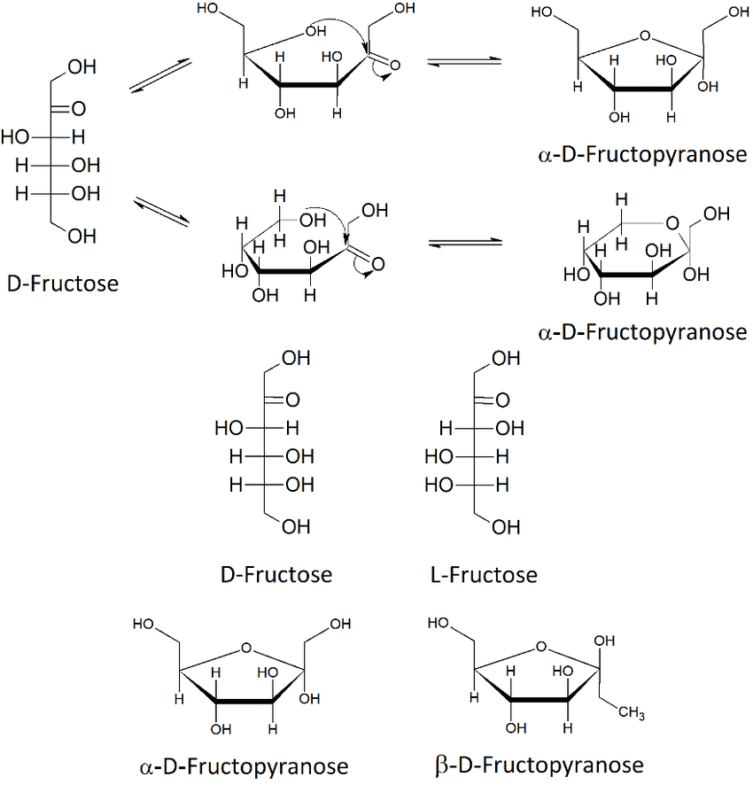
Fig.1. Structural Examples of Alpha-D Fructofuranose.
Source: Adapted from Research Gate.
What are the Common Uses and Applications of α-D-Fructofuranose?
α-D-Fructofuranose’s structure and bioactivity contribute to its importance in food design, nutrition, and industrial uses. For food, α-D provides structural function in many oligosaccharides and polysaccharides, such as inulin, sucrose, and fructooligosaccharides (FOS). These compounds are used in functional foods to act as prebiotics, allowing for the help of the growth of beneficial intestinal bacteria (e.g., Bifidobacterium, Lactobacillus) that support gut health.
In biomedical and pharmaceutical research, α-D-fructofuranose derivatives are model compounds used to study carbohydrate-protein interactions that reveal disease mechanisms and can help create targeting systems for drug delivery. As a result of its specific ring structure and its reactivity, α-D-fructofuranose can be used in glycosylation reactions to help synthesize complex sugar species relevant in developing vaccines and for studies of enzyme mechanisms. α-D-fructofuranose-containing compounds are also used in metabolic studies for insulin response, glycemic control, and digestive resistance, all important factors in the management of diabetes and obesity.
Is α-D-Fructofuranose Safe for Consumption or Use in Lab Settings?
α-D-Fructofuranose and its related derivatives are generally seen as safe for eating or using in a lab setting if the correct purity and regulation are met. When consumed, fructofuranose is naturally found in common sugars such as sucrose and makes up the majority of many functional sweeteners such as fructooligosaccharides (FOS) or difructose anhydrides (DFAs). These sweeteners are non-toxic, non-carcinogenic, and not enzymatically digestible, meaning they can be used to create diabetic-friendly and low-glycemic food products. There has been clinical research that shows that these fructose-based saccharides can be froth by gut microbiota with no known bad effects, thus contributing to gastrointestinal health.
From a laboratory point of view, α-D-fructofuranose is known to be a chemically stable (solid state) and typically a safe material to use and handle in a laboratory environment with standard lab limitations. α-D-fructofuranose is used commonly in biochemical tests, glycosylation studies, and carbohydrate-based synthesis due to its activity and defined structure. Since it is a chemical, α-D-fructofuranose should be used according to a material safety data sheet (MSDS) and standard laboratory safety procedures to minimize risk related to reproductive, eye, or inhalation limits of exposure.
How Can GlycoDepot Help You?
GlycoDepot supports Researchers, Biochemists, and Industry professionals with high-purity, research-grade carbohydrate compounds, including α-D-Fructofuranose. Whether you are involved in glycobiology, metabolism, food technology, or pharmaceutical development, at GlycoDepot, we provide rigorously tested and well-characterized monosaccharides that provide scientists with the accuracy and demands of research. Our product comes with a lot of analytical documentation, including NMR, HPLC, and MS data contributing to the certainty of experimental results. In addition to the product, GlycoDepot provides an exceptional customer service experience by providing technical support, minimum size order quantities, and international shipping, all contributing to your workflow. Any researcher or large industrial customer can determine the quantity they require, and GlycoDepot will provide the product in volumes they require without having to compromise on integrity and quality. Our dedication to truth, science, and belief in international standards is prime in the area of glycochemistry and carbohydrate research. Choosing GlycoDepot for your needs not only means you are purchasing a chemical compound, but it also means you are obtaining a trusted network of knowledge, trust, and innovations, assisting your scientific inquiries and commercial advancement.
FAQs on Monosaccharides
Is α-D-Fructofuranose the Same as Fructose?
Not precisely. Fructose refers to the monosaccharide in a linear form, while the α-D-Fructofuranose is a cyclic form of fructose. In an aqueous environment, fructose exists in a liquid equilibrium of linear, α-D-fructofuranose, and β-fructofuranose.
Can I use α-D-Fructofuranose in my food formulations?
α-D-Fructofuranose and derivatives, like inulin and other DFAs, can be used in food formulations for their sweetness and possible health benefits. However, it is important to check that the specific compound being used has been approved for use in food and to follow regulatory best practices.
What is the difference between α-D-Fructofuranose and β-D-Fructofuranose?
The difference is in the orientation of the hydroxyl group that is attached to the anomeric carbon (carbon 2). In α-D-fructofuranose, the hydroxyl is on the opposite side of the ring compared to the CH₂OH on carbon 5. In β-D-fructofuranose, the hydroxyl is on the same side as the CH₂OH group. These two forms are known as anomers and interconvert in solution.
References:
1: https://www.sciencedirect.com/science/article/abs/pii/S0308814623029540?utm
2: https://www.sciencedirect.com/science/article/pii/S0022030223006896?utm
3: https://pubs.rsc.org/en/content/articlelanding/2012/ra/c2ra00515h
3: Filice, M., & Palomo, J. M. (2012). Monosaccharide derivatives as central scaffolds in the synthesis of glycosylated drugs. RSC Advances, 2(5), 1650–1662.
https://www.sciencedirect.com/topics/chemistry/fructofuranose
Rao, S. T., & Sundaralingam, M. (1998). Confirmation of carbohydrate molecules.
https://www.researchgate.net/figure/Structures-of-the-isomers-and-anomers-of-fructose_fig1_350645582
Oku, T., & Nakamura, S. (2015). Digestion, absorption, fermentation, and metabolism of functional sugar substitutes and their available energy in humans.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4832364
Oku, T., & Nakamura, S. (2015). Digestion, absorption, fermentation, and metabolism of functional sugar substitutes and their available energy in humans.