The characterisation of high-quality monosaccharides has become a main point of advanced glycochemistry, where the development, synthesis, and functional manipulation of carbohydrates play an important role in both fundamental and applied research in the biosciences. Monosaccharides are the critical molecular platform for all complex glycoconjugates such as glycoproteins, glycolipids, and glycosaminoglycans required for various biological processes, including cell recognition, immune signaling, and pathogenesis. The quality or purity of these building blocks plays an important role in glycosylation reactions in which the exact and selective formation of a glycosidic bond is necessary.
In present glycochemistry, high-quality monosaccharides are utilised not only in their application in traditional synthetic carbohydrate chemistry but are also essential in chemoenzymatic synthesis, which uses enzymes such as glycosyltransferases and glycosidases to produce highly selective glycan structures under light conditions. This field has recently advanced with the addition of automated glycan assembly, consisting of fully or semi-protected monosaccharide donors that can be selectively activated and coupled in a programmed manner.
The future development of glycan-based vaccines, cancer immunotherapies, and carbohydrate microarrays for diagnostics also depends on available monosaccharides with known stereochemistry and functional group protection. For researchers who focus on these areas, finding available monosaccharides that meet accurate analytical criteria, including high-resolution NMR, mass spectrometry, and purity attributes, is an extreme data problem for reproducibility and scientific accuracy purposes. Potentially high-purity monosaccharides can also enable the production of synthetic libraries in glycomics studies that are fundamentally transforming our understanding of how sugars manage biological communication and the biological ideas of disease on a molecular level.
What are Monosaccharide Building Blocks?
The Monosaccharides are the simplest building blocks of carbohydrates; they are a single-molecule sugar and the canonical building blocks of more complex carbohydrates (disaccharides, oligosaccharides, and polysaccharides). It also includes those with biological significance, like glucose, fructose, galactose, and mannose, all of which contribute in different ways to cellular biochemical activities in living organisms.
They are made of a linear chain of carbon, hydroxyl groups, and an aldehyde or a ketone, leading to potentially reactive and versatile chemical properties. Monosaccharides are important in glycochemistry because they are building blocks of glycan structures, which are branched structures made by the combination of monosaccharides.
Glycans play important roles in cellular functions and interactions, allowing immune recognition of foreign things, stability of molecules, and the glycosylation of proteins. Because of their small size and reactivity, monosaccharides allow researchers to build specific glycosidic linkages and create complex carbohydrate biosynthetic architectures with biological functions.
The most prominent example of complex carbohydrates is glycoproteins and glycolipids, integral parts of the cell membrane, which depend on monosaccharide subunits to function in roles such as signaling, transport, and immune modulation. Because monosaccharides allow scientists and researchers to synthesize complex biological structures, they are critical components of modern glycoscience research, therapies, and diagnostics. They play a significant role not only in life, they play an immeasurable role in modern applications such as synthetic biology, vaccines, and biopharmaceuticals.
Structural Representation of Monosaccharide Building Blocks
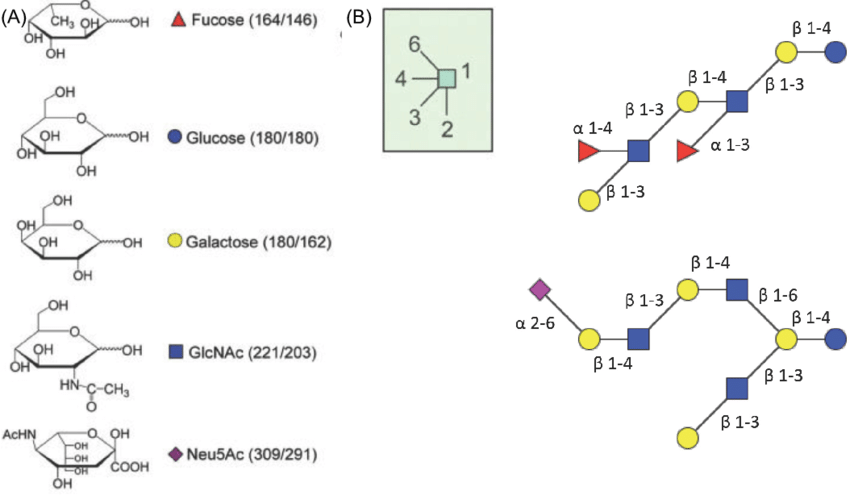
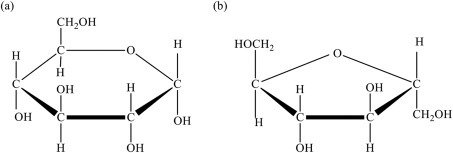
Fig.1 Overview of monosaccharides and their role in forming complex sugars.
Source: Adopted from Research Gate, accessed 2 Jun 2025.
What Are Monosaccharides and Why Are They Important in Glycochemistry
In glycochemistry, monosaccharides are not purely sugars; they are valuable intermediates in the combination of a wide variety and structurally diverse and biologically relevant glycoconjugates, such as glycoproteins, glycolipids, and proteoglycans. These simple carbohydrates that make up the basic unit of all carbohydrates contain multiple reactive hydroxyl groups and can be selectively linked together as glycosidic bonds in synthetic and natural systems.
Monosaccharides can form α and β linkages at various positions on the sugar structure; this allows monosaccharides to be highly flexible, as they can be linked in branched or linear glycan chains with defined stereochemistry and function. In biology, glycans composed of monosaccharides are basic to the regulation of cellular recognition events, including immuno-surveillance, inflammation, and microbial pathogenesis.
For example, on the surface of a mammalian cell, the sialylated glycans serve to determine the half-life of circulating glycoproteins, as well as mediate interactions with immune cells and agent inflammation as needed. Further, mannose-containing glycans are also important in initiating glycoprotein folding and regulating intracellular trafficking through the endoplasmic reticulum and Golgi apparatus.
From a synthetic perspective, monosaccharides are essential for the development of glycomimetic drugs – therapeutic agents that either copy or inhibit carbohydrate-mediated recognition in diseases such as cancer, viral infections, and autoimmune disease. Glycochemists use protected and semi-protected monosaccharide donors to perform regioselective and stereoselective glycosylation, which is a requirement for synthesizing complex oligosaccharide shapes from nature.
In addition, the ability to automate glycan assembly, in conjunction with the evolving enzymatic glycosylation technologies, is only feasible thanks to the availability of detailed monosaccharide building blocks. Research into glycoscience is flourishing, and these base units for sugar are also being utilized in the state-of-the-art high-throughput glycan microarrays in the drive to solve the “sugar code” governing cellular communication and signaling processes.
Ultimately, monosaccharides are critically important in the world of glycochemistry, and serve a greater function than providing something for chemists to build with – they are a mechanism for recognizing basic biochemical processes for explanation. Their use in functional glycobiology points to a reference need for high-purity, structurally defined monosaccharides as a vehicle to proper innovation across multiple domains and facts of immunology, biotechnology, and molecular medicine.
Monosaccharides and Their Importance in Glycochemistry
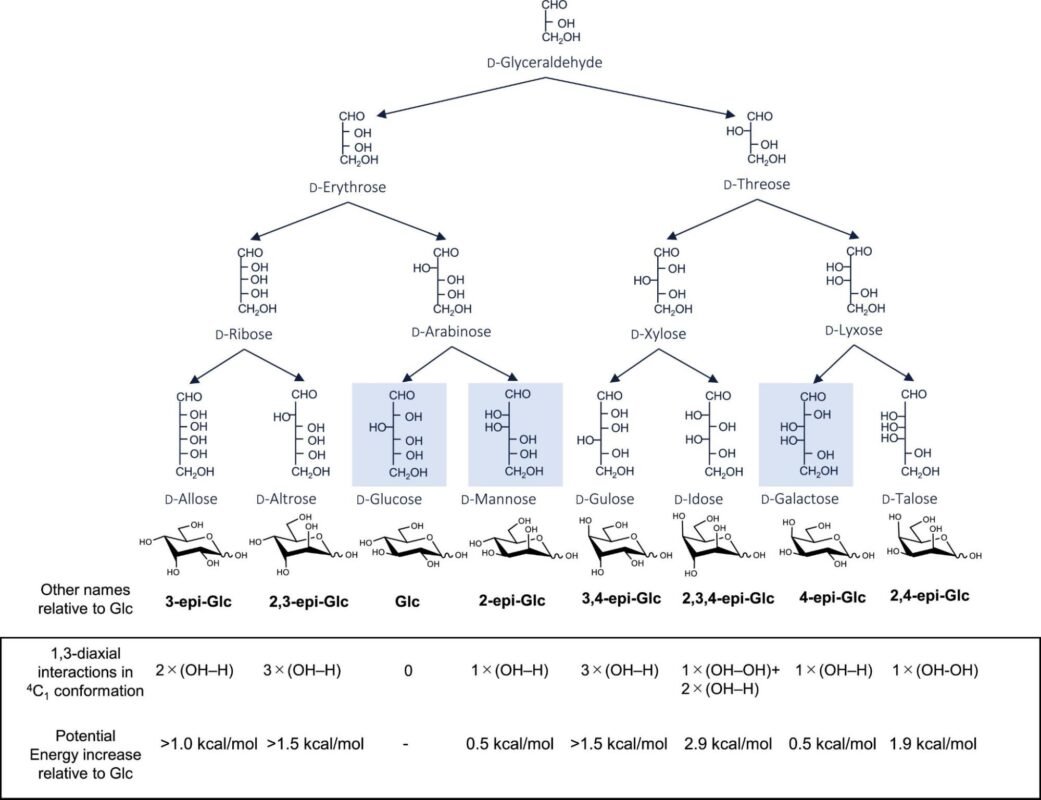
Fig. 1 A visual representation of the crucial roles monosaccharides play in glycochemistry, including energy production, structural formation, and chemical synthesis.
Source: Reproduced with permission from Science Direct.
Buy Different Types of Monosaccharides at Glyco Depot
GlycoDepot provides a scientifically selected range of high-purity monosaccharides specifically developed to enable researchers working with glycochemistry, glycan synthesis, and related biomedical disciplines. Whether you are performing complex glycosylation reactions or trying to recognize the contribution of carbohydrates to cellular responses, our product catalogue of building blocks will meet your research and technical requirements.
Our fully protected monosaccharides are particularly well-suited for directional strategies of glycosylation. These are modified with protecting groups that cover various hydroxyl functionalities to control which sites contribute to glycosidic bonds, and can be used in a fully automated glycan assembly or multi-step oligosaccharide synthesis with precision.
Conversely, our semi-protected monosaccharides are a balance of reactivity and selectivity, combining synthetic flexibility for manipulating defined sterics on a sugar ring while maintaining a protected state on others. These are particularly useful in the context of chemoenzymatic synthesis protocols that can advance one functional group at a time.
Our unprotected monosaccharides offer pre-synthesized options for library-building or rapid-use applications, which are perfect for routine biological glycosylation or simple derivatization. Our monosaccharides are likely to have extensive use in structural carbohydrate studies and can be readily employed in biochemical assays or analytical work.
Every product is promoted by our full characterization data, including NMR spectra, mass spectrometry data, and HPLC chromatograms, to ensure quality, reproducibility, and ultimately confidence in your scientific investigation. We also recognize that every research project is clear. Accordingly, GlycoDepot provides custom synthesis services for rare and not commercially available monosaccharide derivatives in the structure and functional design required by our partners.
Examples of the importance of traditional monosaccharides in science include D-glucose, D-mannose, L-fucose, D-galactose, and N-acetylglucosamine, and many others, all typical foundational sugars to synthetic biology, immunology, cancer research, and diagnostic development. When considering GlycoDepot, researchers are gaining access to quality materials and a platform built on scientific, economic, and analytical transparency.
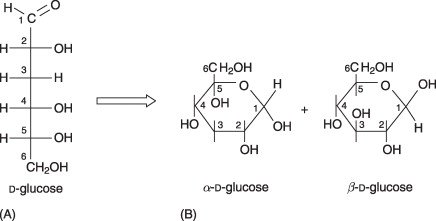
Figure 1. D-glucose molecule shown as (A) open chain and (B) a cyclic pyranose ring in the α and β configurations.
Source: Adapted from Science Direct.
What Is the Monosaccharide Structure?
The monosaccharides can exist as either open-chain (acyclic) or ring (cyclic), with options that depend on the functional groups typically seen (i.e., number of carbon atoms, existence of aldehyde or ketone). In aqueous solutions, monosaccharides exist mainly in their cyclic structure. The cyclic structure is produced by an intramolecular reaction between the carbonyl group and the hydroxyl group. These structures will either be hemiacetals or hemiketals, which will produce a five-membered ring (furanose) or a six-membered ring (pyranose).
When considering the sugar called glucose, it is more likely to exist in its pyranose form, while fructose exists mostly as a furanose form, but can exist as a pyranose.
The ring closure generates another chiral center called the anomeric carbon. At that position, we generate two stereoisomers. There are two types: α-anomer and β-anomer. The orientation of the hydroxyl group at the anomeric position determines the type of anomer. This can be significant during glycosylation reactions since the type of anomer plays an important role in the structure and stability of the glycosidic bond produced.
Basic Structure of Monosaccharide
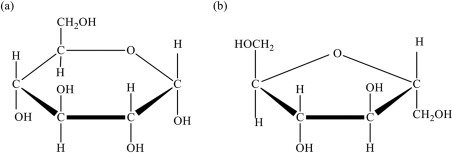
Fig.7.1. Chemical structure of (A) glucose and (B) fructose.
Source: Reproduced with permission from Science Direct.
Chemical Reactions of Monosaccharides
Monosaccharides, as highly reactive molecules due to multiple functional groups, participate in many chemical reactions and these reactions are essential to glycochemistry or synthetic carbohydrate studies. Chemical reactions allow scientists to modify sugar molecules to create complex glycan structures; develop drugs and therapeutics; or to study biological mechanisms.
One of the most important reactions is glycosylation, where monosaccharides undergo a process of acting as a donor or acceptor to form glycosidic bonds. Glycosylation reactions are essential to synthetic oligosaccharides and glycoconjugates like glycoproteins or glycolipids. Glycosylation reactions can be carried out chemically, by using protected derivatives of sugar monosaccharides and activators, or enzymatically by using glycosyltransferases, which allow the formation of products with high regio- and stereoselectivity.
Also, monosaccharides are capable of acetylation, phosphorylation, halogenation, and nitration, each of which alters the reactivity and functional character of the monosaccharide. These reactions allow the introduction of protecting groups, alteration of polarity, or the preparation of the sugars for combination in multistep syntheses.
How Can I Use Monosaccharide Building Blocks in My Research?
Monosaccharides are valuable components for molecular design in many areas within chemical biology and biomedical research, due in part to their structural variety and functional utility. Monosaccharides are the building blocks of glycans, which can be used to produce advanced oligosaccharides and polysaccharides, to help study specifically carbohydrate-protein interactions, cell-surface recognition events, and immune signalling pathways.
Monosaccharides can be converted into various derivatives that can be used in drug design to produce glycosylated drugs, which would typically demonstrate greater water solubility, enhanced stability, and even greater bioavailability. Glycosylation also has the potential to remove or lessen the toxicity of existing compounds, making them safe or more effective for use in therapeutics.
In the case of vaccines, carbohydrate-based antigens are increasingly being produced from synthetic monosaccharide units so that they can mimic structures found on the surface of bacteria or viruses to obtain immune responses in new generations of vaccines.
Monosaccharides are also important to diagnostic materials, especially in the production of glycan microarrays to identify disease-specific biomarkers, and to study lectin-binding profiles associated with cancer, infections, and autoimmune disorders.
How Can GlycoDepot Help You?
GlycoDepot is devoted to promoting glycochemistry research; we are not simply supplying high-quality monosaccharides; we have also set a model, a support system for scientists and developers.
GlycoDepot sources products that are produced under strict quality control standards, assuring the highest purity, reproducibility, and consistency, a must for serious applications such as glycan synthesis, pharmacological development, and diagnostic format development.
Each product is fully documented, with supporting NMR spectra, HPLC topological maps, MS data, and storage instructions. Whether your process means making a complex oligosaccharide or conducting routine assays, documentation is rich and expansive, so you can use the research of innovation as your vehicle.
Each product comes with technical support; we can assist clients with product selection, troubleshooting of methods, and optimization of procedures. Our experienced team has a depth of understanding of the nature of carbohydrate research and how to support researchers at every stage. For specialty needs, GlycoDepot offers custom synthetic services; request unique monosaccharide derivatives with specific protection configurations or stereochemical variations.
FAQs about Monosaccharides
What’s the Difference Between Protected Monosaccharides and Semi-Protected Monosaccharides?
Protected Monosaccharides: All reactive hydroxyl groups are protected; can react selectively at some reaction sites.
Semi-Protected Monosaccharides: Some hydroxyls remain unprotected; depending on the type of protective group on how reactive you want to be and how much control you’re looking for in a synthesis.
How Should I Store and Handle Monosaccharide Reagents?
Store in a cool and dry location, away from sunlight. Follow handling instructions provided to maintain stability and reactivity.
Are Your Products Suitable for Large-Scale Synthesis Projects?
Yes. GlycoDepot provides monosaccharide building blocks in research and bulk-scale formats. Custom bulk orders are available.
References
Reference: Seeberger, P. H., & Werz, D. B. (2007). Synthesis and Medical Applications of Oligosaccharides. Nature, 446(7139), 1046–1051. https://www.nature.com/articles/nature05838.
https://www.sciencedirect.com/science/article/pii/S2667160325000067
Varki, A., et al. (Eds.). (2015). Essentials of Glycobiology (3rd ed.). Cold Spring Harbor Laboratory Press. Chapter: Biological Roles of Glycans. NCBI Bookshelf Link
https://www.sciencedirect.com/topics/medicine-and-dentistry/monosaccharide
Boltje, T. J., Buskas, T., & Boons, G. J. (2009). Opportunities and Challenges in Synthetic Oligosaccharide and Glycoconjugate Research. Nature Chemistry, 1(8), 611–622. DOI: 10.1038/nchem.410
Essentials of Glycobiology (3rd ed.). Varki, A. et al. Cold Spring Harbor Laboratory Press, 2015. Chapter: Monosaccharide Structures. NCBI Link
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/monosacc
Haride
Varki, A. et al. (2015). Essentials of Glycobiology (3rd ed.), Cold Spring Harbor Laboratory Press. NCBI Link
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/monosaccharide
Filice, M., & Palomo, J. M. (2012). Monosaccharide Derivatives as Central Scaffolds in the Synthesis of Glycosylated Drugs. RSC Advances, 2(5), 1729–1742. DOI: 10.1039/C2RA00515H
Reference: Varki, A. et al. (2015). Essentials of Glycobiology (3rd ed.). Cold Spring Harbor Laboratory Press. NCBI Link