Glycans are polysaccharides or complex groups of sugars attached to the outside of our cells, secreted proteins and lipids, and secreted in body fluids such as saliva, milk, and mucus1. These structures of carbohydrates are not simple ornaments. When positioned in highly specific standard forms, they constitute the blood group antigens most of us know as the familiar ABO system, as well as Lewis, P1PK, and others.
Blood group antigens have been investigated for many decades, primarily due to their importance in making blood transfusions and organ transplants safe. However, it has been demonstrated by modern glycoscience that these glycans are anything but mere red blood cell identity labels. They have been considered as molecular signals affecting how our bodies respond to pathogens and how tumors respond or evade the immune system, as well as how the microbiota is formed in our stomach.
Studies have now connected histo-blood group glycans in various ways to health and pathology. Some viruses and other microorganisms, such as bacteria, enter through these as entry points, and thus, blood type affects the risk of contracting an infection. Malignant glycan patterns can be taken over by cancer cells and shield the cancerous cells against immune system attackers. Meanwhile, blood group-related diversity in glycans offers new insights into the concept of personalized medicine, including increasingly accurate diagnostics and therapy design based on a given individual’s glycome.
This blog examines what blood group glycans are, how they are synthesized, and why they are important, other than for transfusion compatibility. Based on the results of an extensive review of the literature on histo-blood group glycans and their biomedical implications, we will demystify their importance in immunity, pathology, and the future of precision healthcare.
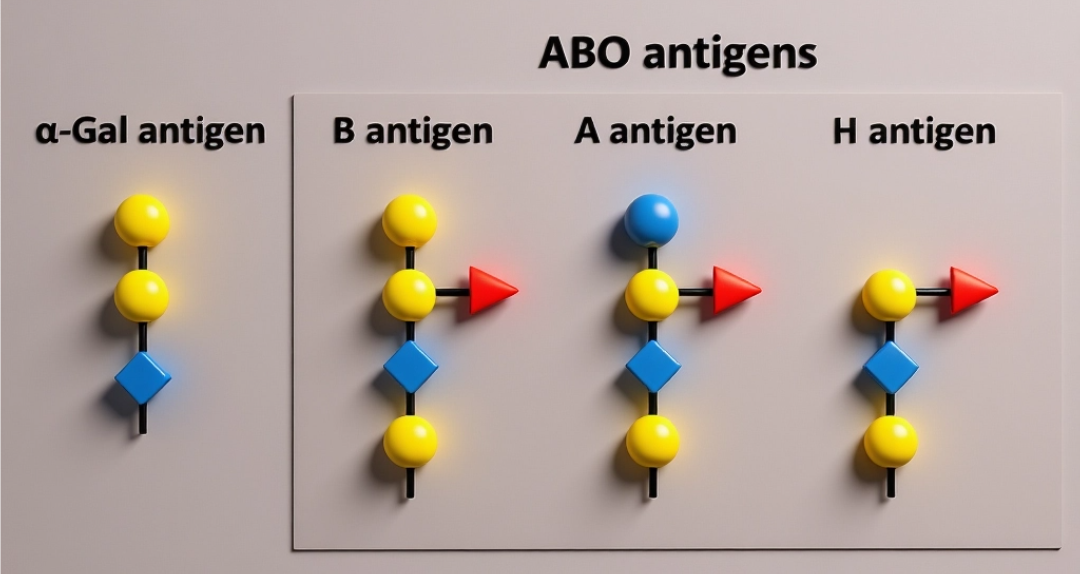
What Are Blood Group Glycans?
Blood group glycans are glycan structures that comprise a unique oligosaccharidic design, a short series of sugar molecules that combine with proteins or lipids on the cells, and are released into the body fluid2. These structures are synthesized in a well-regulated process performed by glycosyltransferase enzymes that add sugars in specific linkages to make up unique patterns.
The most clinically significant is the classic example, the ABO system. The H antigen is almost universally expressed and acts as a precursor structure. Depending upon which glycosyltransferase gene a person is born with, the H antigen can be converted to either an A antigen with the addition of N-acetylgalactosamine or into a B antigen (with the addition of galactose). Inheriting both types of the enzymes causes the affected individuals to express both type A and type B enzymes, whereas those who inherit inactive forms only express the unmodified version of H antigens or blood group O. These minute alterations in sugar are the basis of the well-known A, B, AB, and O blood group phenotypes.
Glycans, however, are by far more than ABO. Other glycan-based blood group systems have also been discovered, each with a characteristic sugar structure and the genes that make the enzymes needed to construct the structure. Candidates include the Lewis system, which is implicated in cell adhesion, immune interactions; the P1PK system, which is implicated in susceptibility to specific infections; the secretor (FUT2) system, which determines the presence of blood group antigens in secretions, including saliva; and the globoside, Forssman, and Sd system, which has a very different pattern and biomedical interest.
A combination of these blood group glycans creates a language written in sugar that is diverse in nature. They are problematic to the structure but very consequential to the physiology, immunity, and even the outcomes of diseases.
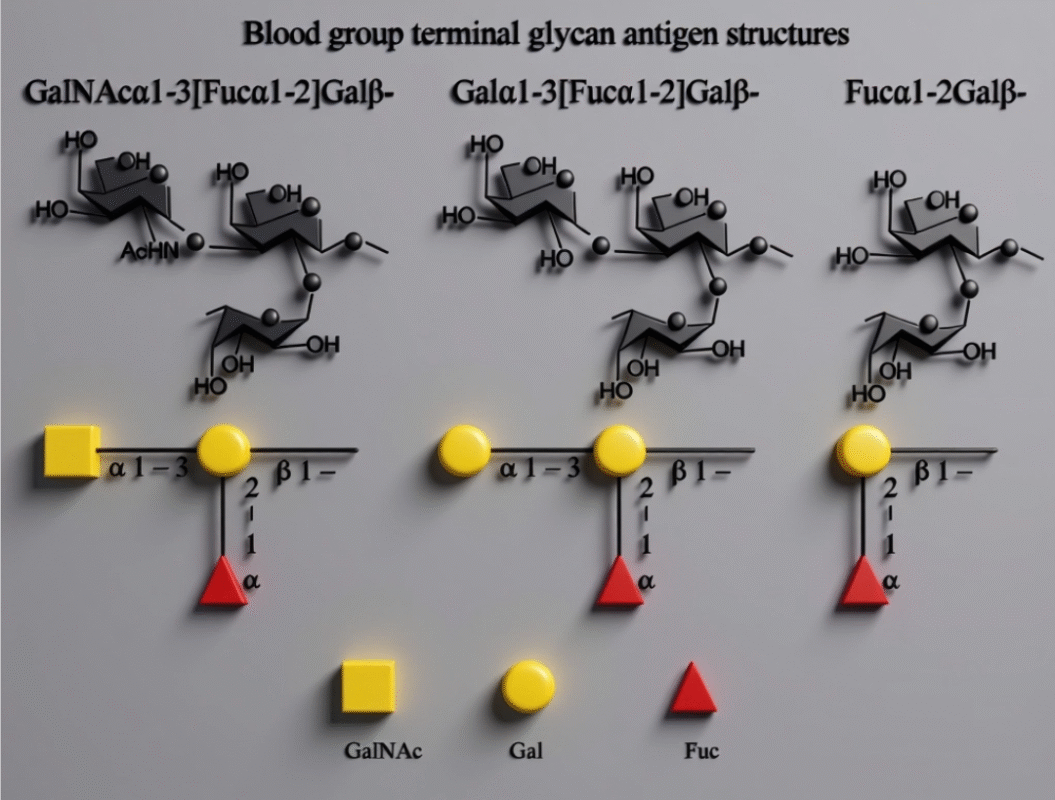
Fig.1. Structural Representation of Blood Group Glycans
How Are These Glycans Synthesized?
The synthesis of blood group glycans is a precisely coordinated process that is catalyzed by glycosyltransferases3. These enzymes are molecular craftsmen, as they add individual units of sugar one by one to a growing chain. What sugars will be added, and in what order, is dependent to a great extent on the genes present in a specific cell or tissue.
In the case of the ABO system, these ABO genes code for the transferases that modify a precursor, the H antigen. The addition of N-acetylgalactosamine will result in an A antigen, where galactose in its place results in a B antigen. In the case in which the enzyme is inactive, the H antigen is not processed, and the result is blood group O.
There are other genes that make other systems of the blood groups. As an example, FUT 1 and FUT 2 encode fucosyltransferases that produce H-type structures in the blood cells and in secretions, respectively. The fucosylation patterns that form the basis of the Lewis system are created by FUT3, whereas other glycosyltransferases govern the production of P1PK, Sd(a), Forssman, and other glycan motifs. Both of the systems represent the distinct interaction between enzyme reaction and sugar substrates.
Particularly, it is not all about genetics that determines the complete glycan profile. Glycan expression is also tissue-specific, dependent upon the availability of sugar donors and physiologically modulated by factors such as infection and cancer. This implies that although a genotype is predetermined, the glycome (a complete set of glycans produced) may differ between tissues and change with time.
Why Blood Group Glycans Matter Beyond Blood Typing
- Infection & microbial interaction
Glycans can be used as pathogen attachment sites. Multiple bacteria, viruses, and parasites can also recognize glycans of the blood type, or their precursors. As an example, noroviruses and some rotaviruses recognize histo-blood group antigens of gut mucins, and this may also account in part why some individuals are resistant to specific viral strains. Likewise, fucosylated milk oligosaccharides associated with maternal secretor phenotype can be used as a decoy receptor to shield breastfed infants against enteric pathogens. These interactions between glycan and pathogen imply that the specifics of blood group glycans determine the odds of infection and disease severity in a population-specific manner.
- Microbiota composition
The sugars adorning the gut lining and that are released to lumenal fluids are food and signals to microbes. Individuals with functional FUT2 (secretors) produce some fucosylated glycans in the mucosal secretions; these bacteria growth-supporting glycans selectively favor the growth of certain commensal bacteria. Non-secretors are found to have a different microbiome profile, and in some cases, those with non-secretors are found to have an increased risk of conditions such as Crohn’s disease. In this way, histo-blood group glycans are at the Weinkauf de facto and topological cusp of host genetics and microbiome ecology.
- Biomarkers and cancer biology
In cancer cells, glycans are frequently remodeled: shortened, sialylated, or in other ways modified glycan structures may appear during malignant transformation. Some of those transformations correspond to blood group structures or their relatives, e.g., sialyl-Lewis antigens (related to Lewis and ABO pathways) are famous tumor-associated glycans and are used clinically; CA19-9 (sialyl-Lewis ahren) is a tumor marker used in pancreatic cancer monitoring. Furthermore, alteration of glycosyltransferase can alter tumor behavior, immune recognition, and metastasis. These glycan shifts have biomarker and therapeutic opportunities.
Clinical And Epidemiological Links
Epidemiology studies have associated blood group phenotypes with the risks of disease in a sometimes consistent manner. The example of group O is that it has been tied to lower chances of severe malaria and, in some analyses, lower chances of very specific cancers. Group A has been implicated in some studies as having an increased risk of certain malignancies in meta-analysis, but this is not consistent. Such associations can be pathogen and population-specific, with some strains of noroviruses following only those individuals with specific histo-blood groups, and secretor status predisposes individuals to certain enteric infections. The implications of these population-level messages predict evolutionary forces such that the varying frequencies of blood group alleles are being buffeted by varying environments.
Mechanistic Insights: How Glycans Change Biology
Two mechanistic themes help explain glycan effects:
- Direct binding and blocking:
When pathogens or toxins bind to a glycan on host cells, they may bind to it as an entry point. On the other hand, the glycans that are soluble are decoys. An obvious protective example is human milk oligosaccharides that act like pathogen receptors.
- Modulation of immune recognition:
Glycans regulate the recognition of cells by antibodies, lectins, in lute, as well as innate immune receptors. Disrupted glycosylation on tumor cells may be able to conceal the antigen or attract inhibitory lectins, which enables immune subversion. On the other hand, carbohydrate-specific antibodies are able to neutralize pathogens or cause autoimmune reactions.
It is through such molecular interactions that glycans become both suspects and instruments of the disease pathways.
Histo-Blood Group Glycans In Precision Medicine
The review contends that histo-blood group glycans are the focus of personalized medicine4. Why? They are inheritable, quantifiable, and subject to functional assessment in infection, cancer, microbiome research, and pharmacotherapeutics. Particular real-life and translational applications are possible:
- Biomarkers: Glycan profiling, when combined with genomic and proteomic data, may be used to make disease classifiers that are stronger than single markers.
- Vaccine design: An understanding of glycan-based pathogen binding would allow informed vaccine design or development of adjuvants, especially those involving priming on histo-blood group antigens used as receptors.
- Therapeutic decoys: It is conceivable that a soluble glycan or glycomimetic could be used to block a pathogen attachment or neutralize a toxin.
- Microbiome-informed nutrition: Personal interventions that take into account secretory status and the availability of glycan may help to shape beneficial microbiota.
The review highlights, however, that such applications are only possible through improved structural characterization, high-throughput glycomics, and a combination of glycomics with other omics data.
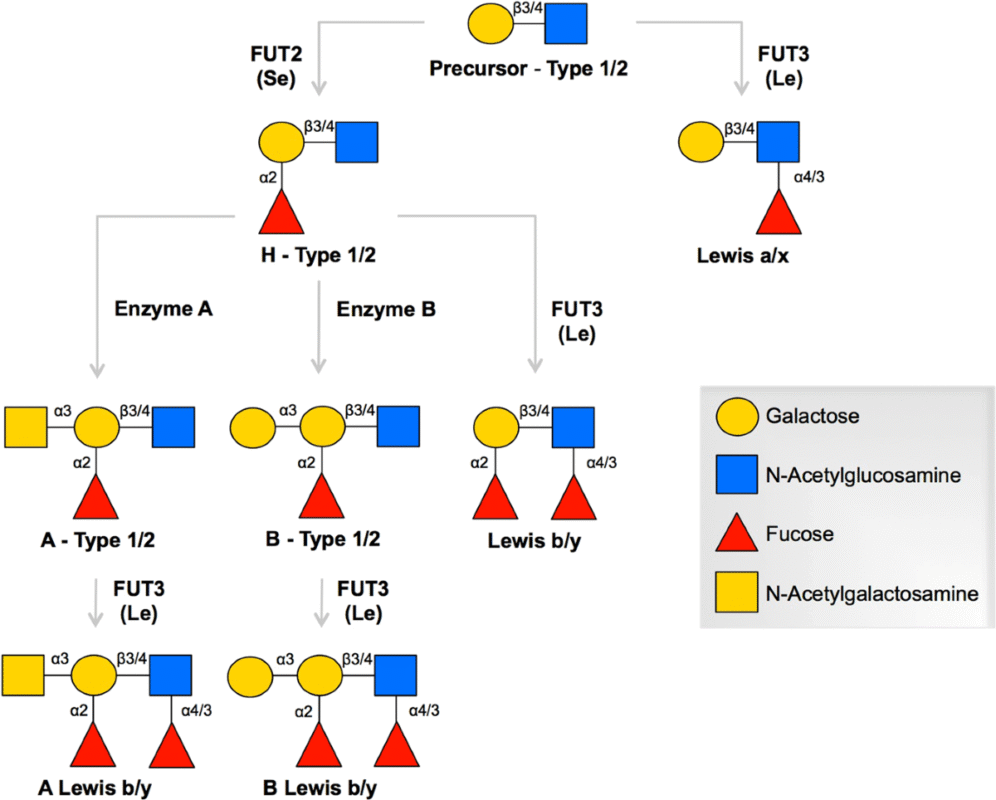
Fig.1. Histo Blood Group Glycans
Recent Advances
An attendant review of the more recent terrain of ABO antigens and differential glycan expression reflects the ongoing attempts to chart glycan heterogeneity between tissues and to interpret the selective pressure that created blood group allele profiles. It reaffirms the importance that glycan expression is dynamic and contextual and that it is linked to pathogen interactions and thrombosis/platelet biology. Collectively, these papers demonstrate a discipline shifting out of a descriptive serology area and towards mechanistic glycoscience with transformative goals.
Practical implications for clinicians and researchers:
- To Clinicians:
Keep in mind that blood group data may present risk information other than transfusion compatibility. As an example, different availability of blood groups within a population could, in infectious outbreaks, help explain frequency patterns of vulnerability.
- For Researchers:
Include glycomic profiling in multi-omics analyses. Combining all three together, genotype, glycan structure, and microbiome will provide insights that cannot be captured when taken in isolation.
In Industry and BioTech: Include glycan-based decoys, glycomimetic drugs, and glycan-conscious vaccines. The translational potential is within the range of anti-infectives to oncology tools.
Limitations And Future Directions
This is a fair assessment made in the review; association is an easy find, but causal mechanisms are not always obvious. Numerous epidemiologic relationships should be replicated in other cohorts. Some native and a few tissue-specific glycomic maps are incomplete. Glycans are notoriously complex to measure: structural isomers, branching structures, and carrier context (lipid vs protein) are important and lead to complexity. To overcome these challenges, standardization of glycomic research methods is necessary, as well as the need to increase cohort size and strict parsing of glycan data with genetic, proteomic, and clinical metadata.
Final thoughts
Blood group glycans are not archaic relics of serology. They are active molecular players in human biology and disease. The reviewed research argues that glycans deserve a central place in precision medicine both as biomarkers and as therapeutic targets. The path forward is clear: invest in better glycomic methods, integrate glycan data with other omics, and design translational studies that test whether manipulating glycan interactions improves outcomes. The sugar code written on our cells is rich with information and potential. We are only beginning to read it.
References:
Dotz, Viktoria, et al. “O-and N-glycosylation of serum immunoglobulin A is associated with IgA nephropathy and glomerular function.” Journal of the American Society of Nephrology 32.10 (2021): 2455-2465.
Jajosky, Ryan Philip, et al. “ABO blood group antigens and differential glycan expression: perspective on the evolution of common human enzyme deficiencies.” Iscience 26.1 (2023).
Stanley, Pamela, et al. “Structures common to different glycans.” (2022).
Dotz, Viktoria, et al. “O-and N-glycosylation of serum immunoglobulin A is associated with IgA nephropathy and glomerular function.” Journal of the American Society of Nephrology 32.10 (2021): 2455-2465.



