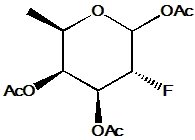
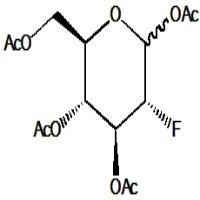
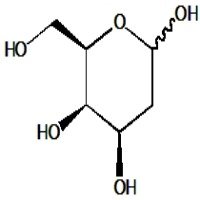
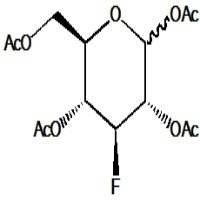
1,3,4-Tri-O-acetyl-2-deoxy-2-fluoro-D-fucose is a synthetic analog of the deoxy sugar fucose, which incorporates a fluorine atom at the 2-position and acetyl protecting groups on hydroxyls at positions 1, 3, and 4. The fluorine substitution at the 2-position significantly changes the sugar’s electronic and steric properties, affecting interactions with enzymes and receptors. Acetyl groups protect selected hydroxyl functions, enabling controlled modification or selective glycosylation. This compound is valuable in chemical glycobiology, especially for studying fucosidase activity and designing potent inhibitors, as the fluorine mimics the enzymatic transition state leading to selective inhibition. Additionally, it aids in synthesizing modified oligosaccharides and glycoconjugates with altered biological functions relevant to cell recognition and immune responses.
IUPAC Name
- (2R,3S,4R,5R)-3,4-Bis(acetyloxy)-5-(acetoxymethyl)-2-fluorotetrahydrofuran
Appearance
- Typically a colorless to white crystalline solid or powder
Source
- Synthetically derived from fucose or related precursors
- Synthesized through multistep deoxygenation, fluorination at C2, and acetylation of hydroxyl groups
Molecular Weight and Structure
- Molecular Weight: 292.26 g/mol
- Molecular Formula: C12H17FO7
- Six-membered fucose ring with fluorine at C2 replacing hydroxyl
- Hydroxyls at 1, 3, and 4 acetylated, with methyl group at C6 characteristic of fucose
Sugar Specificity
- Functions as a modified sugar building block without inherent binding specificity
- Fluorine substitution alters affinity/selectivity when incorporated into larger glycoconjugates
Biological Activity
- May act as a fucosidase inhibitor due to fluorine mimicking enzymatic transition state
- Influences binding affinity/selectivity of glycosides or oligosaccharides toward receptors and enzymes
Purity and Microbial Contamination
- High purity (>95%) required for synthesis and research
- Purity assessed by chromatography and NMR methods
- Microbial contamination negligible in chemical synthesis; sterility needed if for biological use
Identity and Quality Control
- Confirmed via ^1H, ^13C, and ^19F NMR spectroscopy and mass spectrometry
- Quality control includes purity, impurity profile, water content (Karl Fischer), optical rotation
Shelf Life and Storage
- Stable for years if stored dry, protected from moisture/light/air under inert atmosphere at refrigerated temperatures
Application
- Designing potent fucosidase inhibitors
- Mechanistic enzymatic studies of fucosidase action
- Building block for modified oligosaccharide and glycoconjugate synthesis
- Chemical glycobiology and medicinal chemistry research
Key Characteristics
- Fluorinated sugar analog with acetyl protecting groups
- Potent and selective fucosidase inhibition
- Versatile in oligosaccharide synthesis
- Soluble in common organic solvents like chloroform and dichloromethane
- Stable under recommended storage conditions
Citation





Reviews
There are no reviews yet.