What are Carbohydrates?
Carbohydrates are an important class of (Glycosaminoglycans) biomolecules containing carbon, hydrogen, and oxygen as the main components, with the general stoichiometry of (CH₂O)n. Carbohydrates provide the primary source of energy essential for life, and they also have important structural roles. Carbohydrates are present in almost all living things and include simple sugars or monosaccharides (one sugar), double sugars or disaccharides (two sugars), and complex carbohydrates, or polysaccharides, which can be very large polymers made from multiple sugar units.
They are generally regarded as the primary source of fuel, such as glucose, and, in itself, is not only utilized for energy through cellular respiration but also as building blocks for important macromolecules, DNA, and RNA (specifically ribose and deoxyribose sugars). Carbohydrates not only have a role in providing energy but also allow for cell-cell recognition, signaling, and immune responses.
Carbohydrates are found in glycoproteins and glycolipids, which have a carbohydrate component and are needed to communicate with other cells and the environment. Carbohydrates have structural roles, such as cellulose, which is found in the cell walls primarily of plant cells, and chitin, which is found in the exoskeleton of arthropods. In humans and animals, glycogen is a polysaccharide that is a storage form of glucose found primarily in liver and muscle tissue. It quickly provides energy when the body requires it.
Carbohydrates Composition
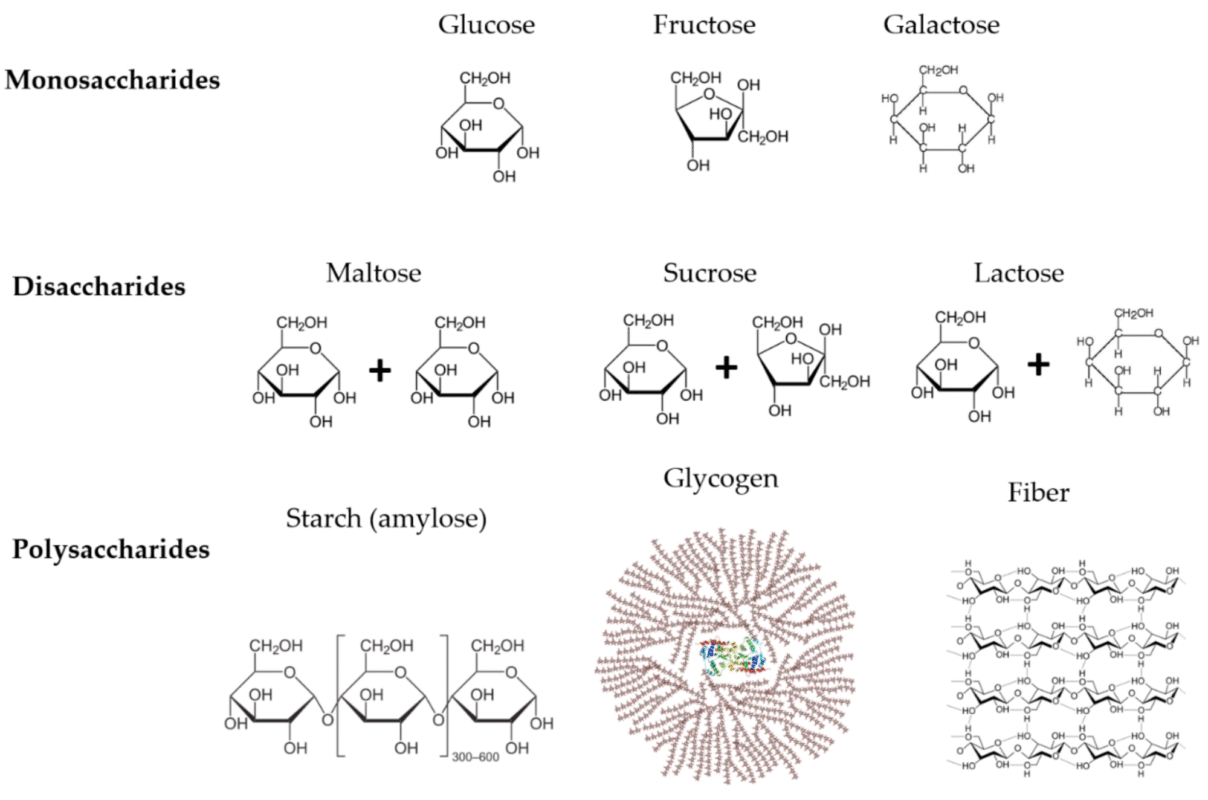
Fig.1. Chemical Composition of Carbohydrates
Source: Image courtesy of mdpi.com
Glycosaminoglycans
Glycosaminoglycans (GAGs) are long linear polysaccharides composed of repeating disaccharides that comprise a uronic acid and an amino sugar. GAG often contains sulfate groups, which makes them highly anionically charged. The negative charge allows GAGs to attract a large amount of water and interact with many proteins, which confer functionality in many different circumstances. GAGs are not only large structural components of the extracellular matrix (ECM) but also exist as glycoconjugates on the membrane of attached cells, contributing to structural support and cell communication.GAGs contribute to hydration, elasticity, and mechanical resistance in tissues of the ECM. Their ability to associate with large amounts of water provides resistance to compressive forces, which are important tissues that undergo mechanical transformation (e.g., cartilage and tendons). Their dual linear flexed state allows GAGs to form linear structures that support the organization of proteins and other ECM components. This key structural support facilitates features associated with the body’s architecture, cellular migratory and differentiative features, and wound healing. In addition to their biological functions, GAGs are also important in cell signaling. GAGs can bind with a variety of signaling molecules, including cytokines, growth factors, chemokines, and others.
Heparan sulfate is one of the most studied GAGs. Heparan sulfate interacts with a variety of signaling factors and acts as a regulator of fibroblast growth factors (FGFs), vascular endothelial growth factors (VEGFs), and transforming growth factor-beta (TGF-β), among others. GAGs modulate and influence biological processes such as angiogenesis, immune response, development, and homeostasis through these binding interactions. GAGs are prevalent in pathological conditions. Pathological conditions in GAG expression or sulfation are associated with human diseases such as cancers, osteoarthritis, fibrosis, and other inflammatory signs. In cancers, when an alteration in heparan sulfate composition occurs, this provides tumor growth, angiogenesis, and metastasis by altering the availability of growth factors and signaling. Overall, in inflammatory conditions, specific GAGs can either promote or inhibit the recruitment of immune cells, and the specific GAG three-dimensional structure can promote or interact with selectins and chemokines.GAGs are now exciting areas of investigation for a variety of therapeutic and biomedical uses. Hyaluronic acid is a non-sulfated GAG widely used for osteoarthritis treatment, wound healing, and aesthetic procedures as it can hydrophilically bind with water and has lubricating properties.
Heparin is another example of a GAG and is known as an anticoagulant, and is used frequently in the clinic. The structural uniqueness of GAGs, specifically their targeted sulfation and chain length, is essential for designing biomimetic materials and targeted drug delivery systems. For this reason, the use of GAGs as an area of research is increasing in importance throughout rehabilitation research in fields including regenerative medicine, biomaterials, tissue engineering, and molecular therapy. The biological complexity of GAGs is further represented by the “sulfation code,” a commonly used characterization of how different regions of sulfation on the GAG chain provide distinct binding specificity to proteins. The sulfation code has a biological consequence for the nature of the protein-GAG interaction. Therefore, emphasis on determining the sulfation code of GAGs has been a major contribution to glycobiology research. Decoding the sulfation code is a powerful opportunity for creating therapeutic possibilities; scientists are aiming to produce synthetic or semi-synthetic GAG substituents that can replicate or inhibit GAG interaction with proteins in a controllable manner.
GAG biosynthesis takes place in the Golgi apparatus and involves a cascade of glycosyltransferases and sulfotransferases that sequentially add and modify GAG chains. Because GAG biosynthesis is not dependent on a template (unlike DNA or RNA synthesis), the GAG chains tend to have considerable structural heterogeneity. This overall complexity is under highly regulated biosynthetic machinery, and a small change in the activity of one enzyme can result in a relatively large alteration in the GAG function. This further signifies the importance of precision by maintaining cellular health and signaling balance.
Basic of Glycosaminoglycans
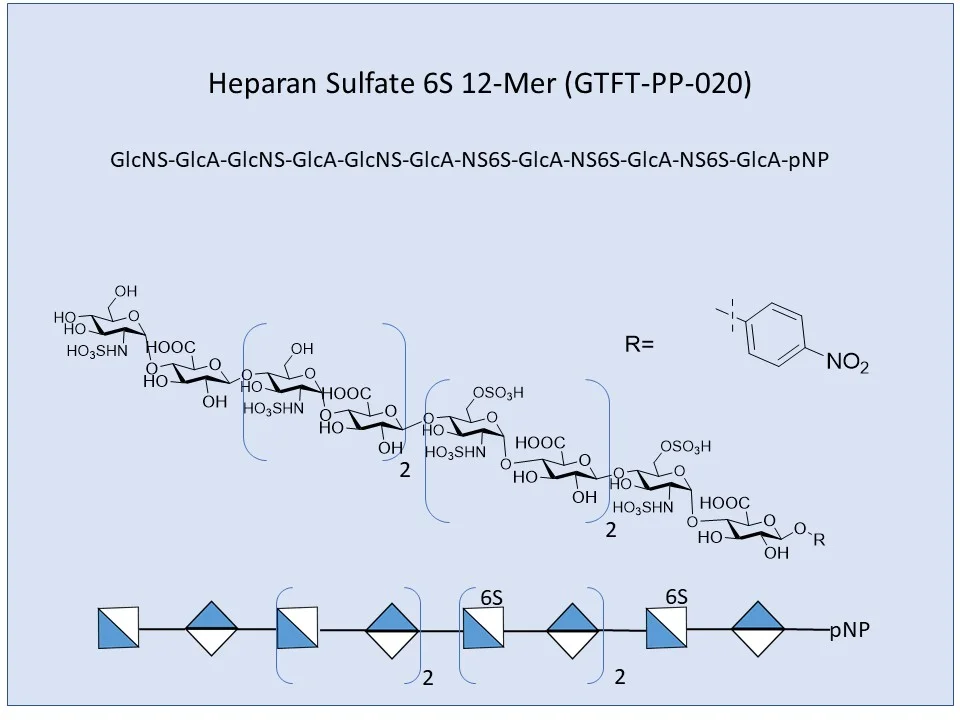
Fig.1. Structure of Glycosaminoglycans
Source: Adopted from glycodepot
Types of Glycosaminoglycans
Glycosaminoglycans (GAGs) can be classified by their repeating disaccharide composition and pattern of sulfation into four general categories: heparin/heparan sulfate (HS), chondroitin/dermatan sulfate (CS/DS), keratan sulfate (KS), and hyaluronic acid (HA). Heparin/HS are highly sulfated and composed of glucuronic or iduronic acid, linked to glucosamine, and constitute an important tissue factor for anticoagulation and the regulation of growth factors. Chondroitin/dermatan sulfate, composed of N‑acetylgalactosamine and uronic acids, contributes to the development of structure in cartilage and plays a role in the wound repair process. Keratan sulfate is made up of dense amounts of galactose and N‑acetylglucosamine and is localized in the cornea, bone, and cartilage, where it helps to withstand compressive stress. Hyaluronic acid has a unique structural distinction from the other GAGs because it is unsulfated and is the only GAG that exists as a free polymer that is not attached to a protein core. The functional role of HA is paramount to the physiological hydration of tissues, and plays an important role in the migration of cells, and importantly, modulates inflammation.
- Heparin / Heparan Sulfate (HS)
Heparin and heparan sulfate are the most sulfated of the GAGs. Structurally, they have the same repeating uronic acid and glucosamine disaccharide unit with variable sulfate groups among the different carbons and amines. Heparin is located primarily in mast cells, and as a result of binding antithrombin III, which inhibits enzymes of the blood coagulation pathway, it is one of the most widely used anticoagulants. Heparan sulfate exists on almost all cell surfaces and basement membranes, where it regulates many biological activities through interaction with growth factors, chemokines, and enzymes. The sulfation of heparan sulfate will likely have multiple roles, as the different sulfations of the heparan sulfate create a “sulfation code” concerning its interaction with the growth factors, chemokines, enzymes, and so forth. This code will dictate angiogenesis, inflammation, and cell proliferation.
- Chondroitin Sulfate / Dermatan Sulfate (CS/DS)
Chondroitin sulfate consists of repeating disaccharides of N-acetylgalactosamine and glucuronic acid. Its main sources are cartilage, tendons, and ligaments. It provides elasticity and mechanical strength to these tissues and is therefore responsible for their primary structural function. Dermatan sulfate is a similar molecule, except iduronic acid replaces glucuronic acid. Dermatan sulfate, as well as chondroitin sulfate, is found in the skin, blood vessels, and heart valves. All CS/DS have indicated roles in the processes of wound healing, remodeling collagen after insult, fibrosis, and as a modulator of growth factors. Depending on the degree of sulfation and structural diversity, CS/DS have heterogeneous biological roles in cell adhesion, organizing extracellular matrices, and tissue responses to injury.
- Keratan Sulfate (KS)
Keratan sulfate contains repeating units of galactose and N-acetylglucosamine in the structure and is classified into three subtypes: KS I (in the cornea), KS II (in cartilage), and KS III (in the brain). Keratan sulfate, unlike other GAGs, does not contain uronic acid; furthermore, most of the sulfation of keratan sulfate occurs at the 6-position of sugars. Keratan sulfate maintains tissue transparency and hydration, especially in the cornea, provides cushioning and shock absorption (e.g., cartilage, cornea), and is involved in developing the nervous system. Keratan sulfate has been connected with neural plasticity and repair as well.
- Hyaluronic Acid (HA)
Hyaluronic acid is the only GAG that is non-sulfate and does not bind to a core protein. Hyaluronic acid has repeating disaccharide units of glucuronic acid and N-acetylglucosamine and exists in the extracellular matrix as a high-molecular-weight polymer. Hyaluronic acid is essential for maintaining the hydration of the tissue, viscoelasticity, and cell migration and proliferation. Hyaluronic acid is important in wound healing, embryonic development, and joint lubrication. Hyaluronic acid biocompatibility, water-binding capacity, etc., bring it to market to the medical and cosmetic world for dermal fillers, eye surgery, and treatments for osteoarthritis
Kinds of Glycosaminoglycans
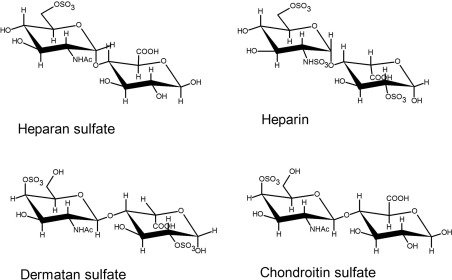
Fig.1. Types of glycosaminoglycans
Source: Adapted from sciencedirect.com
Homopolysaccharides
Homopolysaccharides are polysaccharides that consist of only one type of monosaccharide unit that is repeated in a linear or branched manner. In the case of glycosaminoglycans (GAGs), the only GAG that is a homopolysaccharide is hyaluronic acid (HA). Hyaluronic acid is composed of disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine, both of which come from glucose. Even though each repeating unit has two different sugars, the fact that the repeating units are identical chemically along the chain results in HA being a structural homopolymer for GAGs. Structurally and functionally, HA is distinctly different from the other GAGs not only because it is not sulfated, but also because it does not bind to any protein core. HA has a very high molecular weight and often exceeds millions of Daltons, allowing it to create viscous solutions that contain a large amount of water. This ability to hold water makes HA important for any tissue that needs hydration via water/hyaluronic acid or lubrication due to movement (e.g., synovial fluid, vitreous humor in the eye, and extracellular matrix of skin). Additionally, HA is also important for cell signaling, wound healing, angiogenesis, and tissue regeneration, which is why it is also found in many cosmetic formulations, eye surgeries, and in orthopedic applications.
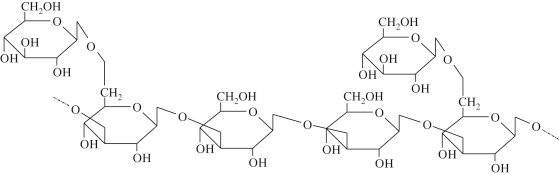
Fig.19.5. Structural Representation of Homopolysaccharides.
Source. Adapted from sciencedirect.com.
Heteropolysaccharides
Heteropolysaccharides are complex carbohydrates that contain two or more types of monosaccharide units organized in repeating disaccharide units. Glycosaminoglycans (GAGs), except hyaluronic acid, are labeled as heteropolysaccharides. GAGs include heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, and keratan sulfate. GAGs are composed of sugar residues in an alternating arrangement, typically an amino sugar (e.g., N-acetylglucosamine, N-acetylgalactosamine) with a uronic acid (e.g., glucuronic acid, iduronic acid). Repeating units (disaccharides) are often heavily modified by sulfation in various places. Sulfation is a structural property of typical heteropolysaccharides, and it is essential to the GAGs’ strong negative charge and interactive ability with proteins, ions, and water molecules.
The sulfation of heteropolysaccharides is very specific and highly regulated. Sulfate groups may be added to the amino sugar, the uronic acid, or both, with often multiple sites on one or both monosaccharides modified. This ability to modify selectively established the so-called “sulfation code”. Sulfation determines GAG binding of certain signaling molecules, growth factors, enzymes, or structural proteins. These binding interactions are critical for mediating biological processes such as inflammation, cell adhesion, blood coagulation, and early embryonic development. For example, the binding of heparan sulfate to fibroblast growth factors (FGFs) to assist in receptor activation is dependent on sulfation. They are complex carbohydrates that contain two or more types of monosaccharide units organized in repeating disaccharide units. Glycosaminoglycans (GAGs), except hyaluronic acid, are labeled as heteropolysaccharides. GAGs include heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, and keratan sulfate. GAGs are composed of sugar residues in an alternating arrangement, typically an amino sugar (e.g., N-acetylglucosamine, N-acetylgalactosamine) with a uronic acid (e.g., glucuronic acid, iduronic acid). Repeating units (disaccharides) are often heavily modified by sulfation in various places. Sulfation is a structural property of typical heteropolysaccharides, and it is essential to the GAGs’ strong negative charge and interactive ability with proteins, ions, and water molecules.
The sulfation of heteropolysaccharides is very specific and highly regulated. Sulfate groups may be added to the amino sugar, the uronic acid, or both, with often multiple sites on one or both monosaccharides modified. This ability to modify selectively established the so-called “sulfation code”. Sulfation determines GAG binding of certain signaling molecules, growth factors, enzymes, or structural proteins. These binding interactions are critical for mediating biological processes such as inflammation, cell adhesion, blood coagulation, and early embryonic development. For example, the binding of heparan sulfate to fibroblast growth factors (FGFs) to assist in receptor activation is dependent on sulfation.
Fig.2.1. Structural Representation of Hetropolysaccharides.
Source. Adapted from ResearchGate.v
Glycosaminoglycans Structure
Glycosaminoglycans (GAGs) are unique due to their linear and highly negatively charged nature, as a result of sulfate and carboxyl functional groups along the repeating disaccharide units. The negatively charged sulfate and carboxyl, believe it or not, lead to strong electrostatic repulsion between adjacent sugar residues that forces the molecule to occupy an extended linear conformation and consequently become rigid instead of curling into compact structures like many polysaccharides do. This is very important for biological function because that linear, stretched-out shape allows GAGs to occupy large volumes when compared relative to their mass, which enables them to retain water to create a gel-like matrix in tissues. The repeating disaccharide units in GAGs generally consist of a uronic acid (either glucuronic or iduronic acid) and an amino sugar (either N-acetylglucosamine or N-acetylgalactosamine). These units are generally heavily sulfated at specific positions depending on the type of GAG and the enzymes responsible for GAG synthesis. The negative charges from sulfuric acid or carboxylic acid groups do not just give the sugar its hydrophilic property, they allow GAGs to ionic bond with proteins that are positively charged because of their amino charges, I.e., growth factors, cytokines, enzymes, and structural matrix proteins.
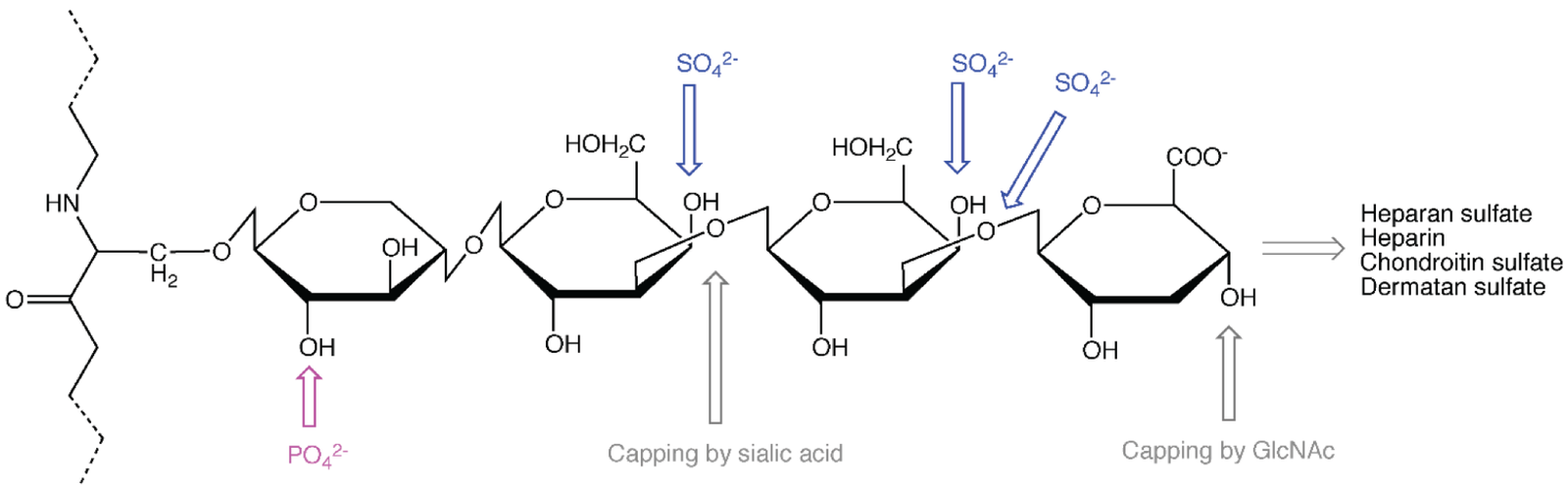
Source. Adapted from mdpi.com
Glycosaminoglycans Function
Glycosaminoglycans (GAGs) have many important biological functions, which can be attributed to their unusual structural properties, specifically their linear, extended nature and the presence of high negative charge. One of the main functions of GAGs includes water retention/hydration of the tissue. Because GAGs are so hydrophilic, they can attract and hold massive amounts of water, forming viscous gels within the extracellular matrix (ECM). The gel-like consistency of ECM allows for proper tissue turgor (the amount of water in connective tissues) and elasticity, and this is sculpted in the cartilaginous tissues of joints and the connective tissue of skin (as well as in the vitreous humor of the eye). Hydration is particularly critical to support the biomechanical properties of turgidity in these tissues. GAGs also provide powerful mechanical strength and structural support to connective tissues. GAGs provide the ECM with the ability to withstand compressive forces when providing polymerization and cross-linking with fiber proteins, such as collagen and elastin. For instance, collagen, keratin sulfate, and chondroitin sulfates are part of the aggrecan complex of cartilage that allow cartilage to be an effective, flexible, and responsive “shock absorber” of a moving body.
Heparin
Heparin is a highly sulfated glycosaminoglycan (GAG) with potent anticoagulant function and is the most negatively charged biomolecule present in nature. It consists of repeating disaccharide units, typically consisting of iduronic acid and glucosamine residues, in which the abundance of sulfate groups has been modified and contributes to its strong negative charge and allowing for the interaction with many different positively charged proteins and ions. In contrast to the other GAGs that are secreted into the extracellular matrix, heparin is mainly produced and stored intracellularly in mast cell secretory granules, particularly in tissues in contact with the external environment, such as the lungs, liver, and gastrointestinal tract. Upon activation of the mast cell or injury, heparin can be released and enter circulation for its anticoagulant properties.
Chondroitin Sulfate
Chondroitin sulfate is a major glycosaminoglycan (GAG) that is widely found in the extracellular matrix of connective tissues, and in the greatest abundance in cartilage, tendons, ligaments, and skin. It is a structural unit that consists of repeating disaccharides of N-acetylgalactosamine and glucuronic acid, with varying degrees of sulfation and pattern of sulfation, at the 4 and/or 6 position of the galactosamine residue. These sulfate groups confer a negative charge to the molecule, creating an environment that allows the GAGs to not only attract water but also attract cations. This property is important in maintaining the structure and biomechanical properties of tissues. Chondroitin sulfate, along with the core proteins, combine to formulate large proteoglycan complexes by forming a synthetic attachment point with the core proteins, to aggrecan, for example. The binding of proteoglycans with the matrix allows the cartilage to resist compressive stresses that develop in articular cartilage. Chondroitin sulfate binds water in the hydration of the matrix and, combined with the ability to separate aggrecan and other proteoglycans, provides a gel-like matrix that reduces stress and allows for recovery of a joint after it has been mechanically stressed. This property is particularly beneficial in weight-bearing joints such as the knee and hip joints.
Keratan Sulfate
Keratan sulfate is a distinct glycosaminoglycan that is made up of repeated disaccharide segments typically involving galactose and N-acetylglucosamine that can sometimes be sulfated at various positions. Keratan sulfate differs from other GAGs in that keratan sulfate does not involve uronic acid, resulting in structural and functional characteristics similar to and dissimilar from other GAGs. However, keratan sulfate is mostly found in the cornea, cartilage, and bone, where keratan sulfate is an important component in maintaining tissue hydration and transparency. In the cornea, keratan sulfate acts to regulate water levels for clarity and toughness. In cartilage and bone, keratan sulfate acts as one component of the compressive resilience of the extracellular matrix by both retaining water and giving compressive strength. Keratan sulfate interacts with core proteins such as lumican and keratocan to assist with tissue development and tissue healing, and repair.
Hyaluronic Acid (HA)
Hyaluronic acid (HA) stands out as a glycosaminoglycan that is non-sulfated and has a very high molecular weight. Composed of repeating units of glucuronic acid and N-acetylglucosamine, HA is found widely throughout connective, epithelial, and neural tissues and is important for tissue hydration due to its capacity to bind and retain water, and to expand in volume, when it is hydrated – important in the skin, eyes, and joints. Hyaluronic acid is biologically active, among other things, in joint lubrication (notably, by reducing friction between articular cartilages), in wound healing, and in tissue regeneration. In synovial fluid, HA maintains synovial fluid viscosity while permitting movement. In dermatology and cosmetic medicine, the formulation of dermal fillers is based on the viscoelastic properties and biocompatibility of HA. At the site of wounds, HA promotes cellular migration and proliferation, contributing to healing wound sites more quickly.
Applications of Glycosaminoglycans
Glycosaminoglycans (GAGs) are potent biological polymers in many medical and therapeutic applications because of their endogenous sources, biocompatibility of natural polymers, water binding capacity, their ability to interact with cellular macromolecules, and their often are structurally complex with populations that can support many biological functions. GAGs are applicable for clinical intervention and biomedical research.
Anticoagulation
Heparin is one of the most widely known GAGs, and it is helpful as an anticoagulant for surgeries, hemodialysis, and to treat thromboembolic disorders. Heparin enhances the activity of antithrombin III and inhibits the blood clotting factors such as thrombin and factor, therefore preventing blood clotting. Heparin is useful for systemized antifactor Xa or antithrombin therapy as unfractionated, low molecular weight, or depot formulations.
Joint Therapy
Hyaluronic Acid (HA) and Chondroitin Sulfate (CS) are commonly used for patients with osteoarthritis and degeneration of joints. HA injections, often referred to as viscosupplementation, restore the viscoelasticity of synovial fluid, mitigating pain and improving mobility. CS supplements are thought to improve joint health by preventing cartilage breakdown and inflammation, thereby making them popular dietary additive options in some forms of joint health supplements.
Tissue Engineering
For regenerative medicine, GAGs are integral to biomaterial scaffolds. GAG-based scaffolds serve as useful biomaterials because they are those components include GAGs that can bind many growth factors and regulate cellular behavior. Furthermore, the GAG-based scaffolds help improve adhesion, proliferation, and differentiation of cells. GAG-based scaffolds are useful for the repair and regeneration of cartilage, skin, and nervous tissue. Furthermore, new techniques to engineer bioengineered organ systems and wound dressings use GAG-based scaffolds.
Glycosaminoglycan Effect:
Glycosaminoglycans (GAGs) have many effects on biological tissues and cellular processes, in large part due to their high negative charge density and linear and flexible structure. Both the structural properties of GAGs and the ability of GAGs to attract water and interact with many proteins make GAGs vital for the functional performance of the tissue and the capacity to respond to complex physiological signals. GAGs can hydrate tissues and absorb mechanical force and stress. This is particularly important in cartilage, skin, and the eye, where GAGs absorb shock, stress, and efficiently maintain structure even when stressed. In the extracellular matrix (ECM), GAGs control protein signaling and positive and negative protein interactions by binding to secreted factors, including cytokines, chemokines, and growth factors, resulting in direct effects on inflammation, tissue repair, and tumor growth. From a clinical point of view, their biological properties provide a basis for support for anticoagulant activity (chemostasis) such as that offered by heparin, and could also support anti-inflammatory or even antitumor effects. Their promiscuity and structural properties interact to highlight many potential medical applications and benefits, notably concerning wound healing, cancer, and drug delivery.
Glycosaminoglycan FAQs
Where are glycosaminoglycans distributed in the body?
They are debtly distributed in the extracellular matrix (ECM) component of a tissue, on cell surface receptors, and there are considerable amounts in cartilage, synovial fluid, cornea, and bone.
What is the difference between proteoglycans and glycosaminoglycans?
Proteoglycans consist of protein cores that are covalently attached to glycosaminoglycan chains via a tetrasaccharide linker; glycosaminoglycans alone are considered linear polysaccharides.
What is the significance of glycosaminoglycans?
Glycosaminoglycans are significant in maintaining hydration and structural integrity of tissues, and mediate signalling pathways and healing, lubrication, and homeostasis processes.
What and how are glycosaminoglycans constructed?
Glycosaminoglycans are large molecules composed of repeating disaccharide units, usually made from one uronic acid (glucuronic or iduronic acid) and one amino sugar (glucosamine or galactosamine). Keratan has galactose instead of uronic acid.
What are the constituents of glycosaminoglycans?
Same as above- consists of a long chain of disaccharide repeat units, extensively sulfated (except hyaluronic acid).
Are glycosaminoglycans homopolysaccharides?
No-the only homopolysaccharide in the glycosaminoglycan family is hyaluronate; all others in the family are heteropolysaccharides, containing a variation of different monosaccharide units and sulfation.
References:
Chizhov, Alexander O. “Complex Carbohydrates and Glycoconjugates: Structure, Functions and Applications.” International Journal of Molecular Sciences 22.22 (2021): 12219.
https://www.mdpi.com/1422-0067/22/22/12219
Ricard‐Blum, Sylvie, et al. “A biological guide to glycosaminoglycans: current perspectives and pending questions.” The FEBS Journal 291.15 (2024): 3331-3366.
https://pubmed.ncbi.nlm.nih.gov/38500384
Ricard‐Blum, Sylvie, et al. “A biological guide to glycosaminoglycans: current perspectives and pending questions.” The FEBS Journal 291.15 (2024): 3331-3366.
https://pubmed.ncbi.nlm.nih.gov/38500384
Ricard‐Blum, Sylvie, et al. “A biological guide to glycosaminoglycans: current perspectives and pending questions.” The FEBS Journal 291.15 (2024): 3331-3366.
https://pubmed.ncbi.nlm.nih.gov/38500384
Ricard‐Blum, Sylvie, Romain R. Vivès, Liliana Schaefer, Martin Götte, Rosetta Merline, Alberto Passi, Paraskevi Heldin et al. “A biological guide to glycosaminoglycans: current perspectives and pending questions.” The FEBS Journal 291, no. 15 (2024): 3331-3366.
https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.17107
Ricard‐Blum, S., Vivès, R.R., Schaefer, L., Götte, M., Merline, R., Passi, A., Heldin, P., Magalhães, A., Reis, C.A., Skandalis, S.S. and Karamanos, N.K., 2024. A biological guide to glycosaminoglycans: current perspectives and pending questions. The FEBS Journal, 291(15), pp.3331-3366.



