Request a quote
| Appearance : | White amorphous powder (salt-free), lyophilized |
|---|---|
| Activity : | Grade Ⅰ 30 U/mg-solid or more |
| Stability : | Stable at −20 ℃ for at least one year |
|---|---|
| Molecular weight : | approx. 70,0001) |
| Appearance | White amorphous powder (salt-free), lyophilized |
|---|---|
| Activity | GradeⅠ 30 U/mg-solid or more |
| Stability | Stable at −20 ℃ for at least one year(Fig.1) |
|---|---|
| Molecular weight | approx. 70,0001) |
| Michaelis constants1) | 11 ± 1.1×10-4 M (Maltose), 3.6 ± 0.51×10-4 M (Maltotriose), 2.5 ± 0.33×10-4 M (Maltotetraose), 1.6 ± 0.02×10-4 M (Maltopentaose) |
| Structure | Glycoprotein[E 280 nm1 cm (1 %)=14.5] 1 cm |
| Optimum pH | 4.5−5.0(Fig.3) |
| Optimum temperature | 60 ℃(Fig.4) |
| pH Stability | pH 4.0−8.5 (25 ℃, 20 hr)(Fig.5) |
| Thermal stability | below 45 ℃ (pH 5.5, 10 min)(Fig.6) |
| Substrate specificity1,2) | This enzyme completely hydrolyzes soluble starch, amylopectin, glycogen,α-orβ-limit dextrin, amylose, maltooligosaccharides and panose. |
This enzyme is useful for structural analysis of carbohydrates and for enzymatic determination of α-amylase in combination with the related enzymes in clinical analysis.

The formation of glucose is measured with reducing sugar as the index, by the modified Fehling-Lehmann-Schoorl method.
One unit causes the formation of ten milligrams of glucose in 30 minutes under the conditions detailed below.
| A. Starch solution | 1.0 %: Suspend 1.0 g of soluble starch (Merck) in 90 mL of H2O, dissolve by boiling for 3 min, and cool to room temperature. Add 5.0 mL of 1.0 M acetate buffer, pH 4.5, and make up to 100 mL with H2O (should be freshly prepared). |
|---|---|
| B. Alkaline solution | 100 g of NaOH, 365 g of potassium sodium tartrate tetrahydrate, per 1,000 mL of H2O |
| C. CuSO4 Solution | 7.0 % :70 g CuSO4・5H2O/1,000 mL of H2O |
| D. KI solution | 30 %: 300 g of KI per 1,000 mL of H2O (store in a brownish bottle) |
| E. H2SO4 Solution | 25 % |
| F. Na2S2O3 Solution | 50 mM: 49.638 g pf Na2S2O3・5H2O, 4.0 g of Na2CO3 (stabilizer) in 4,000 mL of H2O (store in a brownish bottle and keep for 3~4 days before use). |
| G. Enzyme diluent | 10 mM acetate buffer, pH 4.5 |
1.Pipette 4.0 mL of substrate solution (A) into a test tube (32 φ× 200 mm) and equilibrate at 40 ℃ for approximately 5minutes.
| Concentration in assay mixture | |
|---|---|
| Acetate buffer | 42 mM |
| Starch | 0.8 % |
2.Add 1.0 mL of the enzyme solution* and mix.
3.After exactly 15 minutes at 40 ℃, add 2.0 mL of alkaline solution (B) to stop the reaction.
At the same time, prepare the blank by first mixing the substrate solution with 2.0 mL of alkaline solution after incubation for 15 min at 40 ℃, followed by addition of the enzyme solution.
4.Add 2.0 mL of CuSO4 solution (C) and, after covering the test tube with a marble (40 mmφ) to prevent evaporation, place the test tube in a boiling water bath.
5.After 20 minutes, remove the test tube from the boiling water bath and cool to room temperature under running water.
6.Add 2.0 mL each of KI solution (D) and H2SO4 solution (E) in this order.
7.Shake the test tube and determine the amount of residual Cu2+ by titration with Na2S2O3 solution (F).
8.Record the titers (mL) of the test (Δt) and the blank (Δb), and calculate the titration difference in mL (Δ sample: Δb−Δt).
*Dissolve the enzyme preparation in ice-cold distilled water and dilute to 0.4−1.5 U/mL with enzyme diluent (G), immediately before assay.
Activity can be calculated by using the following formula :
Volume activity (U/mL) =
Δsample×30 min×df
Δglucose×15 min
=
Δsample
Δglucose
×2.0×df
Weight activity (U/mg) = (U/mL)×1/C
| Δglucose | : Titration difference (mL) for ten miligrams of glucose (Determine the titration difference by using glucose standard solution (5.0mg/mL) instead of the enzyme solution under the above assay conditions.) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
1)K.Hiromi, Y. Nitta, C.Numata and S.Ono; Biochim.Biophys.Acta, 302, 362 (1973).
2)J.Fukumoto; Protein, Nucleic Acid and Enzyme, 4, 3 (1959).
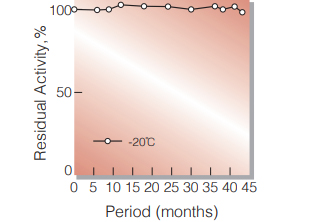
Fig.1. Stability (Powder form)
(kept under dry conditions)
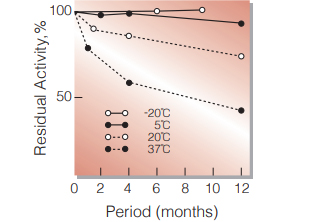
Fig.2. Stability (Powder form)
(kept under dry conditions)
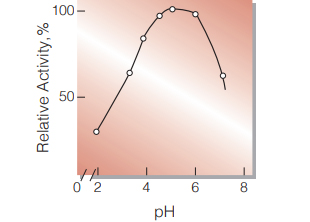
Fig.3. pH-Activity
40℃,15min-reaction in 50mM buffer solution: pH2.0,sodium acetate-HCI; pH3.0-6.0,acetate;pH6.0-7.0, phosphate
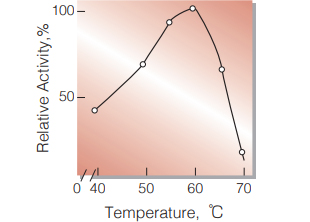
Fig.4.Temperature activity
15min-reaction in 50mM acetate buffer,pH4.5
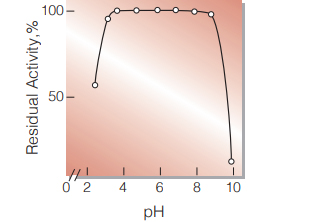
Fig.5.pH-Stability
25℃,20hr-treatment with 50mM buffer solution: pH3.0-6.0 acetate; pH6.0-9.0,phosphate;pH9.0-10.0, borate
| Size | 1 MG, 10 MG, 5 MG |
|---|
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
|
|
|
0% |
10 in stock
10 in stock
10 in stock
Gal-a1,3-(Fuc-a1,2)-Gal-b1,4-Glc, CAS:59957-92-5, G06873PC
10 in stock
Not a member? Create an account
Already got an account? Sign in here
Reviews
There are no reviews yet.