

Request a quote
| Appearance : | Yellowish amorphous powder, lyophilized | ||
|---|---|---|---|
| Activity : | GradeⅠ | 180 U/mg-solid or more | |
| GradeⅡ | 100 U/mg-solid or more | ||
| (containing approx. 50 % of stabilizers) | |||
| Contaminant : | Catalase | GradeⅠ | ≤ 5.0×10-3 % |
| Grade Ⅱ | ≤ 3.0 % | ||
| Stabilizers : | Potassium gluconate, sodium glutamate | ||
| Appearance | Yellowish amorphous powder, lyophilized | ||
|---|---|---|---|
| Activity | GradeⅠ | 180 U/mg-solid or more | |
| GradeⅡ | 100 U/mg-solid or more | ||
| (containing approx. 50 % of stabilizers) | |||
| Contaminant | Catalase | GradeⅠ | ≤ 5.0×10-3 % |
| GradeⅡ | ≤ 3.0 % | ||
| Stabilizers | Potassium gluconate, sodium glutamate | ||
| Stability | Stable at −20 ℃ for at least one year(Fig.1) | |
|---|---|---|
| Molecular weight | approx. 153,000 | |
| Michaelis constants | 3.3×10-2 M (β-D-Glucose),3) | |
| 6.1×10-2 M (2-Deoxyglucose) | ||
| Structure | Glycoprotein with 2 moles of FAD | |
| Inhibitors | p-Chloromercuribenzoate, heavy metal ions (Cu2+, Hg2+, Ag+) | |
| Optimum pH | 4.5(Fig.3) | |
| Optimum temperature | 40 − 50 ℃(Fig.4) | |
| pH Stability | pH 4.5−6.0 (30 ℃, 20 hr)(Fig.5) | |
| Thermal stability | below 50 ℃ (pH 5.7, 1 hr)(Fig.6) | |
| Substrate specificty | (Table 1) | |
| Effect of various chemicals | (Table 2) | |
This enzyme is useful for enzymatic determination of glucose, and for an amylase-activity assay in combination with α-glucosidase (AGH-211, if maltooligosaccharide or modified starch is used as a substrate), in clinical analysis.
![]()
4-AA : 4-Aminoantipyrine
EHSPT : N-Ethyl-N-(2-hydroxy-3-sulfopropyl)-m-toluidine
The formation of quinoneimine dye is measured at 555 nm by spectrophotometry.
One unit catalyzes the formation of one micromole of hydrogen peroxide (half a micromole of quinoneimine dye) per minute under the conditions detailed below.
| A. MES-Na buffer pH 5.7 | 0.1 M: Dissolve 2.13 g of 2-(N-morpholino) ethansulfonic acid (MW = 213.25) in approx. 60 mL of H2O and, after adjusting the pH to 5.7 with 1 N NaOH at 25℃, make up to 100 mL with H2O (stable at 5℃ for 1 month). | |
|---|---|---|
| B. Glucose solution | 15 %: Dissolve 1.5 g of β-D-glucose in H2O, and make up to 10 mL, at least 2 hours before, but on the same day as, the assay. | |
| C. 4-AA solution | 0.5 %: 50 mg of 4-aminoantipyrine (MW = 203.25) / 10 mL of H2O (stable at 5℃ in a brownish bottle for at least 1 week). | |
| D. EHSPT solution | 40 mM: 118 mg of N-ethyl-N-(2-hydroxy-3-sulfopropyl)-m-toluidine (MW = 295.3) / 10 mL of H2O (stable at 5℃ in a brownish bottle for at least 1 week). | |
| E. Peroxidase solution | 500 purpurogalin units (U) /mL H2O | |
| F. Enzyme diluent | 10 mM MES-Na buffer, pH 5.7, containing 0.1 % Triton X-100 | |
1.Prepare the following working solution in a brownish bottle and store on ice.
| (Should be prepared fresh) | ||
| 30 mL | Buffer solution | (A) |
| 6.0 mL | Substrate solution | (B) |
| 0.3 mL | 4-AA solution | (C) |
| 0.3 mL | EHSPT solution | (D) |
| 0.3 mL | POD solution | (E) |
| Concentration in assay mixture | |
|---|---|
| MES buffer | 79 mM |
| D-glucose | 131 mM |
| 4-AA | 0.2 mM |
| EHSPT | 0.3 mM |
| POD | ca.4 U/ml |
2.Pipette 3.0 mL of working solution into a cuvette (d = 1.0cm) and equilibrate at 37 ℃ for approximately 5 minutes.
3.Add 0.1 mL of the enzyme solution* and mix by gentle inversion.
4.Record the increase in optical density at 555 nm against water for 2 to 3 minutes with a spectrophotometer thermostated at 37 ℃, and calculate the ΔOD per minute from the initial linear portion of the curve (ΔOD test).
At the same time, measure the blank rate (ΔOD blank) using the same method as the test, except that enzyme diluent (F) is added instead of the enzyme solution.
*Dissolve the enzyme preparation in ice cold enzyme diluent (F) and dilute to 0.05−0.2 U/mL with the same buffer, immediately before the assay.
Activity can be calculated by using the following formula :
Volume activity (U/mL) =
ΔOD/min (ΔOD test−ΔOD blank)×Vt×df
32.8×1/2×1.0×Vs
= ΔOD/min×1.89×df
Weight activity (U/mg) = (U/mL)×1/C
| Vt | : Total volume (3.1 mL) |
| Vs | : Sample volume (0.1 mL) |
| 32.8 | : Millimolar extinction coefficient of quinoneimine dye under the assay conditions (cm2/micromole) |
| 1/2 | : Factor based on the fact that one mole of H2O2 produces a half of quinoneimine dye. |
| 1.0 | : Light path length (cm) |
| df | : Dilution factor |
| C | : Enzyme concentration in dissolution (c mg/mL) |
1)The Enzymes, Vol.ⅫB, P.421 (P.D.Boyer, ed.), Academic Press (1975).
2)Method in Enzymology, Vol.Ⅸ, p.82 (S.P.Colowick and N.O.Kaplan, ed.),Academic Press (1966).
3)B.E.P.Swoboda and V.Massay; J.Biol.Chem., 240, 2209 (1965).
4)P.J.Auses, S.L.Cook and J.T.Maloy; Anal.Chem., 47, 244 (1975).
5)D.C.Williams, G.F.Huff and W.R.Gaitz; Clin.Chem., 22, 372 (1976).
[0.1M of Substrate, 79mM MES buffer, pH 5.7, at 30 ℃ ]
| Substrate (0.1M) | Relative activity(%) |
|---|---|
| D-Glucose | 100 |
| 2-Dexy-D-glucose | 16.2 |
| Glucono-1,5-lactone | 0.06 |
| L-Glucose | 0.00 |
| Galactose | 3.10 |
| Mannose | 2.10 |
| Substrate (0.1M) | Relative activity(%) |
|---|---|
| Fructose | 0.24 |
| Xylose | 0.93 |
| Ribose | 0.00 |
| Maltose | 0.69 |
| Lactose | 0.00 |
[The enzyme dissolved in 0.1M MES buffer, pH 5.7 (10 U/mL) was incubated with each chemical for 2 hr at 25 ℃.]
| Chemical | Concn.(mM) | Residual activity(%) |
|---|---|---|
| None | – | 100 |
| Metal salt | 2.0 | |
| MgCl2 | 92.6 | |
| CaCl2 | 93.6 | |
| BaCl2 | 94.4 | |
| CoCl2 | 98.1 | |
| MnCl2 | 95.1 | |
| ZnSO2 | 94.3 | |
| FeCl2 | 96.8 | |
| NiCl2 | 91.7 | |
| CuSO4 | 71.6 | |
| AgNO3 | 58.6 | |
| HgCl2 | 0.7 | |
| PCMB | 2.0 | 31.6 |
| MIA | 2.0 | 96.8 |
| NaF | 2.0 | 97.1 |
| Chemical | Concn.(mM) | Residual activity(%) |
|---|---|---|
| NaN3 | 20 | 96.3 |
| EDTA | 5.0 | 97.3 |
| o-Phenanthroline | 2.0 | 95.3 |
| α,α′-Dipyridyl | 2.0 | 99.5 |
| Borate | 50.0 | 96.1 |
| IAA | 2.0 | 96.1 |
| MIA | 2.0 | 101.1 |
| Hydroxylamine | 10.0 | 98.3 |
| Sodium bisulfite | 10.0 | 100.0 |
| hydrazine | 10.0 | 103.1 |
| Triton X-100 | 0.1 % | 111.2 |
| Brij 35 | 0.1 % | 108.0 |
| Tween 20 | 0.1 % | 110.7 |
| Span 20 | 0.1 % | 106.7 |
| Na-Cholate | 0.1 % | 106.1 |
| SDS | 0.1 % | 113.1 |
PCMB, p-Chloromercuribenzoate; MIA, Monoiodoacetate; EDTA, Ethylenediamimetetraacetate; IAA, Iodoacetamide; NEM, N-Ethylmaleimide; SDS, Sodium dodecyl sulfate.
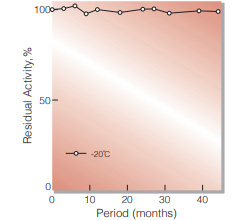
Fig.1. Stability (Powder form)
(kept under dry conditions)
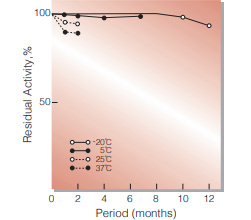
Fig.2. Stability (Powder form)
(kept under dry conditions)
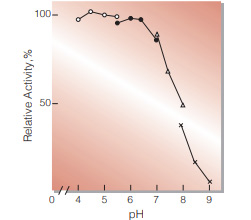
Fig.3. pH-Activity
37 ℃, 5min-reaction in 79 mM buffer solution : ○̶̶○ . acetate;●̶̶● MES;△̶̶△, BES;×̶̶×, BICINE
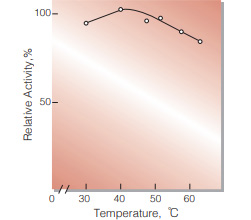
Fig.4. Temperature activity
(5 min-reaction in 79 mM MES buffer, pH5.7)
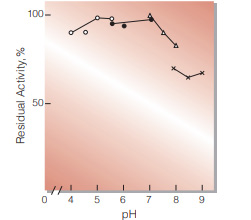
Fig.5. pH-Stability
30 ℃, 20hr-treatment with 0.1 M buffer solution : ○̶̶○ . acetate : ●̶̶● MES : △̶̶△, BES : ×̶̶×, BICINE
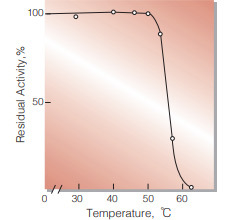
Fig.6. Thermal stability
(1hr-treatment in 79 mM MES buffer , pH5.7)
| Size | 1 MG, 10 MG, 5 MG |
|---|
| 0% | ||
| 0% | ||
| 0% | ||
| 0% | ||
| 0% |
| Name | α1,4-galactosyltransferase; LgtC |
| Catalog Number | EN01006 |
| E.C. | 2.4.1.228 |
| Product Description | E. coli recombinant α1,4-galactosyltransferase from Neisseria meningitides |
| Unit Definition | One unit is defined as the amount of enzyme that catalyzes the formation of 1 µmol of Galα1,4Lac from UDP-Gal and Lactose per minute at 37 °C. |
10 in stock
| Name | UDP-sugar pyrophosphorylase;BIUSP |
| Product Code | EN01019 |
| E.C. | 2.7.7.64 |
| Product Description | E. coli recombinant UDP-sugar pyrophosphorylase from Bifidobacterium longum |
| Unit Definition | One unit is defined as the amount of enzyme that catalyzes the formation of 1 μmol of UDP-Gal from Gal-1-P and UTP per minute at 37 °C. |
10 in stock
| Name | α2,3-sialyltransferase; PmST1 |
| Catalog Number | EN01002 |
| E.C. | 2.4.99.4 |
| Product Description | E. coli recombinant α2,3-sialyltransferase from Pasteurella multocida (P-1059) |
| Unit Definition | One unit is defined as the amount of enzyme that catalyzes the formation of 1 µmol Siaα2,3Lac from CMP-Sia and lactose per minute at 37 °C. |
10 in stock
| Name | L-fucokinase-GDP-fucose pyrophos-phorylase;FKP |
| Product Code | EN01016 |
| E.C. | 2.7.1.52/2.7.7.30 |
| Product Description | E. coli recombinant L-fucokinase/GDP-fucose pyrophosphorylase from Bacteroides fragilis |
| Unit Definition | One unit is defined as the amount of enzyme that catalyzes the formation of 1 μmol of Fuc-1-P from L-Fuc and ATP per minute at 37 °C. |
10 in stock
Not a member? Create an account
Already got an account? Sign in here
Reviews
There are no reviews yet.